About this course:
This nursing continuing education course is designed to update nurses on current knowledge and guidelines for patients experiencing arthritic disease with a focus on osteoarthritis (OA), rheumatoid arthritis (RA), psoriatic arthritis, axial spondyloarthritis, gout, and juvenile arthritis.
Course preview
Purpose: This nursing continuing education course is designed to update nurses on current knowledge and guidelines for patients experiencing arthritic disease with a focus on osteoarthritis (OA), rheumatoid arthritis (RA), psoriatic arthritis, axial spondyloarthritis, gout, and juvenile arthritis. After completing this activity, learners will be prepared to:
- differentiate among the types of arthritis by clinical presentation and treatment
- distinguish the role of pathophysiology in the development of symptoms in patients experiencing arthritis, including OA, RA, psoriatic arthritis, axial spondyloarthritis, gout, and juvenile arthritis
- compare and contrast risk factors for the development of each of the described forms of arthritis
- identify findings of diagnostic procedures used for arthritis that indicate specific arthritic disorders
- plan patient education for and implementation of treatment regimens used for various forms of arthritis
- identify nursing interventions to meet the specific needs of patients with each form of arthritis
- interpret current research trends in arthritis that are expected to impact the future incidence, severity, and treatment of arthritis
Arthritis is the leading cause of disability in the US. Currently, nearly 60 million adults and 300,000 children in the US are impacted by arthritis in their joints and organs. Arthritis affects 1 in every 4 adults. However, it is projected that every 5 years, arthritis cases will increase by 3 million; by 2040, it is anticipated that 78 million adults will experience arthritis. Although there are over 100 arthritis-related conditions, the most common are osteoarthritis (OA), rheumatoid arthritis (RA), psoriatic arthritis, axial spondyloarthritis, gout, and juvenile arthritis (Arthritis Foundation, 2022a; Centers for Disease Control and Prevention [CDC], 2022).
Anatomy and Physiology
The human body contains bones, cartilage, and joints; these facilitate movement. Joints that do not move are called synarthrosis; those with limited movements, such as the symphysis joint, are referred to as amphiarthrosis. A diarthrosis joint freely moves; such joints include ball-and-socket, pivot, hinge, saddle joint, plane joint, and condyloid joint (see Figure 1). These joints are classified as synovial joints and contain articular cartilage on the surface of the bones (see Figure 2). Articular cartilage contains connective tissue with an extracellular matrix comprised of water, proteoglycans, ground substance, and collagen. Amino acids and disaccharides are found in the proteoglycans allowing for elasticity, stiffness, and movement. The joint capsule is a fibrous connective tissue that covers and stabilizes the joint. The synovial membrane surrounds the joint capsule and is responsible for secreting synovial fluid, which serves as a buffer in preventing friction or rubbing of the bones, protecting them during movement. Synovial fluid is also housed in bursas, which allow the tendons to move freely. Synovial fluid and articular cartilage collaborate in reducing friction in the joint throughout mobility. Inflammation or wearing down of any part of the joint can contribute to the discomfort associated with arthritis (Scanlon & Sanders, 2019).
Figure 1
Different Kinds of Joints
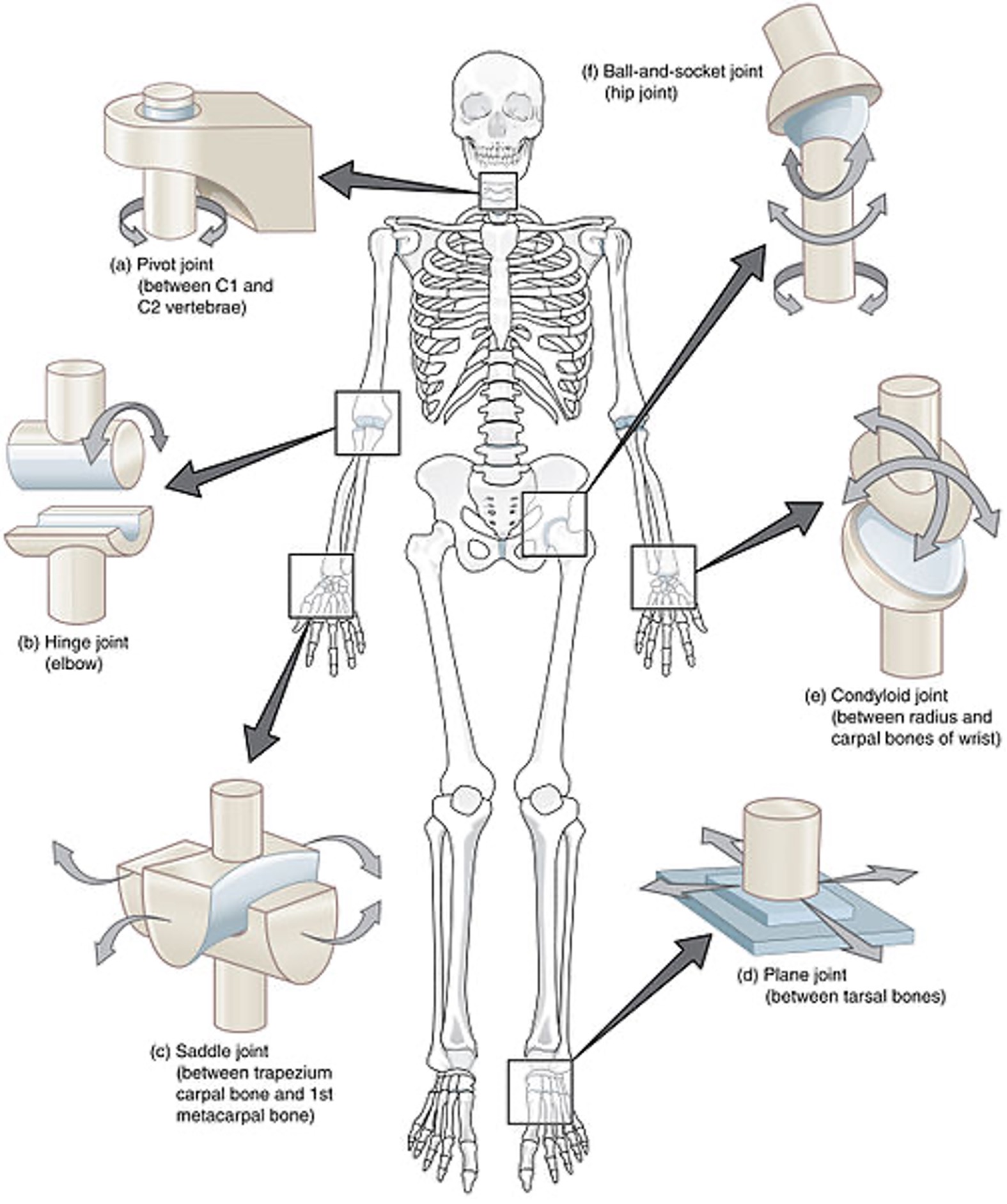
(Open Stax College, 2013b)
Figure 2
Close-Up of Joint
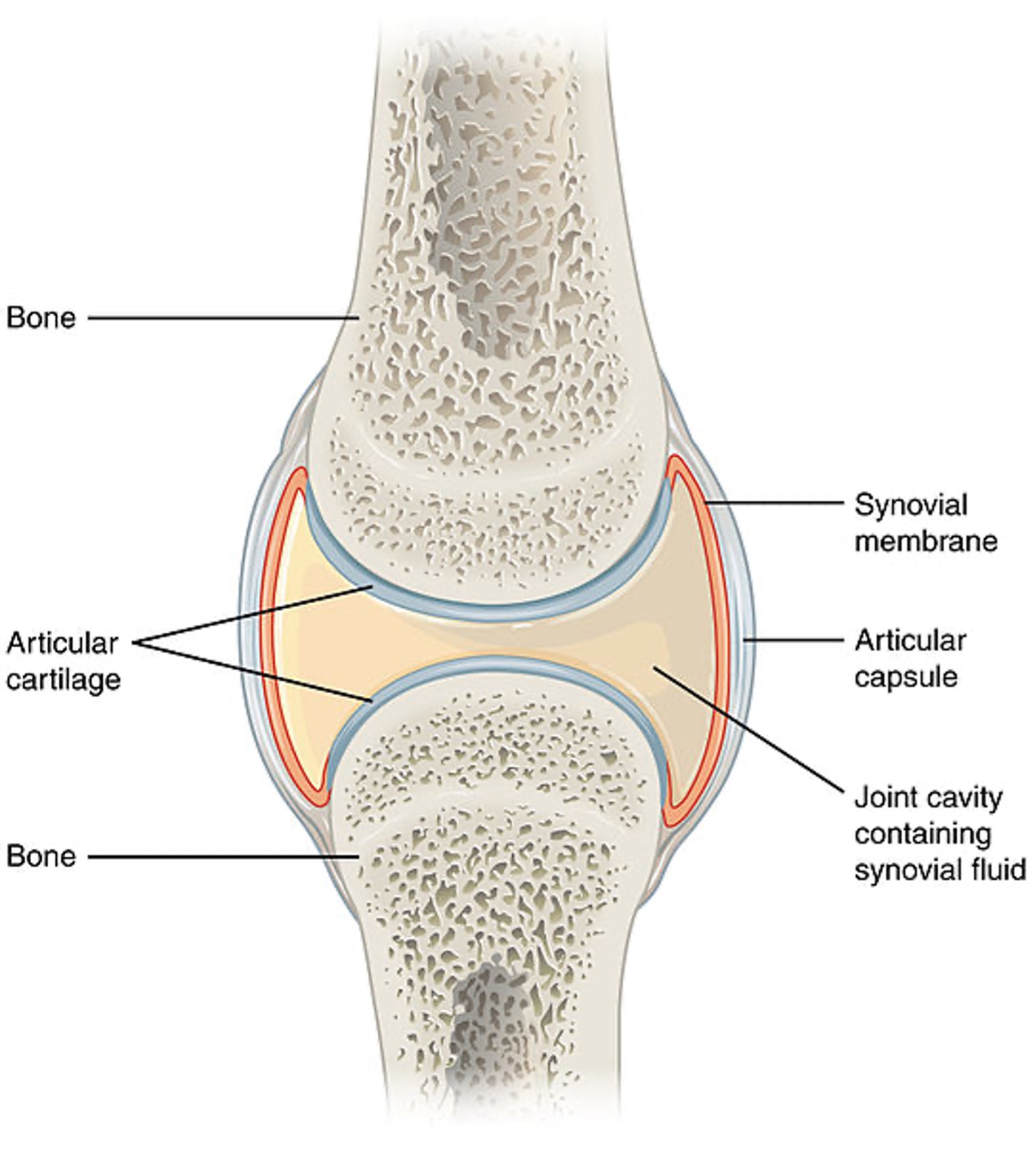
(Open Stax College, 2013a)
Osteoarthritis
OA alters the entire joint due to biological, chemical, and viscoelastic changes in the tendons and ligaments. The changes occur gradually as the articular cartilage ages. As the articular cartilage is worn down, the cushioning is decreased, and the protection of the joint is lost. Once this occurs, or if the articular cartilage is damaged, osteophytes form---causing pain and reduced range of motion (ROM). Osteophytes are also known as bone spurs. In addition, the joint's surface becomes rough, causing the patient stiffness and instability with movement.
When the damage begins, the cartilage will have more water and proteoglycans, weakening the collagen. Cytokines are released, causing further joint damage. The disease may be primary or idiopathic, with no precipitating illness related to the OA, or secondary due to previous joint injury, overuse, or inflammation. OA may be further complicated by the development of Bouchard’s nodes and overproduction of synovial fluid, exacerbating pain and restricting movement. As this occurs, the patient may use their affected digit or extremity less, causing atrophy in the neighboring muscles and further weakness. Ligaments may become edematous and fibrotic, which continues to potentiate the pain and limit movement. The large and weight-bearing joints are most affected: knees, hips, and ankles (Hinkle & Cheever, 2018; Hoffman & Sullivan, 2020; Norris, 2019, 2020; Scanlon & Sanders, 2019).
Risk Factors/Protective Features
OA is ubiquitous; currently, it impacts nearly 27 million people in the US. Individuals at risk for OA tend to be older females with obesity who use their weight-bearing joints extensively, including within their occupation. Most individuals experience structural changes in their third or fourth decade despite not experiencing symptoms (Hinkle & Cheever, 2018). Typically, occupations that require frequent bending down and repetitive motion of the knees, hands, or hips can contribute to the development of OA. Patients with OA may have played sports, sustained a previous injury or muscle weakness, be genetically predisposed, have a history of inflammatory arthritis, and/or other bone and joint disorders. As individuals age, the rates of OA increase; it is more prevalent in non-Hispanic black women than non-Hispanic white women and more frequently seen in the hands of women than men (Hoffman & Sullivan, 2020; Norris, 2020).
Signs and Symptoms
OA worsens over time; pain, decreased ROM, tenderness to touch, edema, deformity, and joint instability are experienced more often as the disease progresses. Patients may report a decrease in pain while at rest; however, patients may also experience pain during the night and morning stiffness in their joints. The pain is often aching and difficult to localize. In addition, a patient may experience crepitus and grinding in their joint due to the breakdown of cartilage (Hoffman & Sullivan, 2020; Norris, 2020).
<
...purchase below to continue the course
Diagnosing OA requires a comprehensive history and physical exam. Initially, the patient will typically present with pain and stiffness at the site; from there, the healthcare provider conducts a physical assessment to identify alterations in mobility, strength, and size of the joint(s). If morning stiffness lasts more than 30 minutes, the patient should have a further evaluation for OA. The provider may suggest serum blood tests to rule out an inflammatory process. Radiographs often serve as confirmatory agents. Joint-space narrowing, subchondral sclerosis or cysts, formation of spikes on the tibial eminence, or visible osteophytes may be noted on the x-rays (Hoffman & Sullivan, 2020; Norris, 2020).
Treatment/Management
Nonpharmacological
Caring for a patient with OA requires the nurse to embrace a holistic approach to treatment and management (see table 1). Patients may receive physical and occupational therapy to prevent further disability related to their OA. Exercise and muscle-strengthening activities are helpful to patients with OA. Warm and cold compress therapy may relieve pain. Patients may utilize assistive devices, such as a cane or walker, to help with mobility. If weight-bearing joints, such as the knees, are impacted, overweight patients may benefit from participating in a weight loss program. If nonpharmacological measures coupled with medications do not help the patient, surgical joint replacement, surgical fusions, arthroscopic irrigation, and debridement may be utilized (Hoffman & Sullivan, 2020; Norris, 2020).
Pharmacological
Most medication management in a patient with OA focuses on pain management and reducing inflammation (see Table 1). Patients may be given acetaminophen (Tylenol) to combat prostaglandins from furthering inflammation. In addition, nonsteroidal anti-inflammatory drugs (NSAIDs) may be utilized when acetaminophen (Tylenol) is no longer effective in treating the patient’s pain. NSAIDs work by inhibiting cyclooxygenase, thus preventing prostaglandin activity. NSAIDs may not be given if a patient has a history of gastrointestinal (GI) bleeding or renal insufficiency; NSAIDs may increase the risk of cardiac or neurological events, potentiate renal injury, and cause bleeding stomach ulcers. If oral medications are ineffective in reducing pain, patients may be given intra-articular corticosteroid or hyaluronan injections. Corticosteroids decrease inflammation; hyaluronan increases lubrication on articular surfaces. If a patient is experiencing a joint effusion, intra-articular corticosteroids are helpful. Opioid medications are a last resort due to the risk of dependency and abuse and are not intended for chronic pain management (Hoffman & Sullivan, 2020; Norris, 2020).
Table 1
Nursing Care of the OA Patient
CLINICAL PRACTICE AREA | ASSESSMENT | INTERVENTION |
Clinic setting (the most common location for encounters with OA patients) | Patient’s preferred activity Daily activity level Weight and height Gait: observe for posture and symmetry of movement Muscle strength and ROM Occupational risk: does the patient’s job require lifting, squatting, bending, or repetitive activity? The onset of unilateral joint pain The onset of crepitus The presence of co-morbidities: non-insulin-dependent diabetes mellitus NIDDM, cardiovascular disease (CVD), depression Joint specific discomfort and limitation; exemplar: thumb arthritis | Teach the patient to self-manage activity by exploring and incorporating preferred activities (Schlenk & Shi, 2019). Teach to include weight-bearing activity within daily routine with a goal of 6000 – 10000 steps (Skou et al., 2018). Promote the maintenance of recommended weight or reduction for obese patients (Lewis et al., 2019). Refer for further evaluation if malalignment is noted and the patient presents bow-legged or knock-kneed, as these increase the risk for more rapid progression of OA (Goldman & Schafer, 2016). Implement 0-5 scale (5 = normal, 0 = no strength for both measures. Implement specific measures to support joint (i.e., medially-directed patellar taping for the knee; Schlenck & Shi, 2019) Teach the patient to use supports (i.e., a knee brace or back support). Assure proper placement. Refer to physical therapy (PT) for specific exercises for strengthening and proper positioning (Skou et al., 2018). Teach that interventions can prevent progression and relieve pain; a diagnosis of OA does not mean inevitable progression and constant pain (Goldman & Schafer, 2016). Teach that occasional sounds in joints are expected but to report if frequency increases (Lo et al., 2017). Identify factors that may decrease activity levels and plan holistically for the individual patient to maximize function and prevent complications (Schlenk & Shi, 2019). Gentle exercise and applying ice and heat for five to ten minutes (note decrease time for smaller joints) on the thumb provide direct comfort (Mayo Clinical Health Letter, 2018). |
Perioperative | Frequency and dose of NSAIDs Postoperative mobility | Educate the patient to discontinue aspirin (Bayer) or NSAIDs 1 week before undergoing any procedure with an increased risk for bleeding. For low-risk surgeries, no discontinuation is needed (Burchum & Rosenthal, 2019). Plan early and progressive ambulation for all postoperative patients (Lewis et al., 2017) |
Obstetrics | Over-the-counter (OTC) use of NSAIDs | Teach that NSAIDs interfere with prostaglandin formation and increase the risk of patent ductus; thus, NSAIDs should be avoided during the last trimester (Burchum & Rosenthal, 2019) |
Pediatrics | Participation in sports or intense activity Use of seat belts | Encourage the utilization of joint support measures (i.e., knee support for basketball) to decrease long-term injury risk (Schlenk & Shi, 2019) Teach proper seat belt use to prevent skeletal injuries, specifically knee injuries caused by bracing during motor vehicle accidents. |
Home Health | Environmental safety Utilization of supportive mobility devices | Perform fall risk assessment and implement measures to minimize tripping hazards inside the home (Pechar, 2020). Teach the proper use and evaluate the effectiveness of canes and walkers, where indicated. |
Rehabilitation | Pain level and mobility after joint replacement | Assist in progressive distance and duration of ambulation; promote assistive devices such as zipper pullers and long-handled shoehorns (Schenck & Shi, 2019) |
Geriatrics | Pain level after resting and during moderate activity Activities of daily living | Encourage participation in individual and group activities to improve mobility (Schub & Schiebel, 2017). Implement adjunct measures such as plate guards to promote independence and delegate tasks to staff for maximal function (Schlenk & Shi, 2019). |
Adult acute medical-surgical | Joint mobility with passive ROM (PROM) and active ROM (AROM) Address changing nutritional needs Assess nursing implications for OTC and prescribed medications | Ambulate the patient. Perform ROM every 2 hours if the patient cannot perform independently (Ignatavicius, 2019). Collaborate with a dietitian to plan a high-fiber, high-protein diet to promote elimination and decrease muscle loss during immobility (Ignatavicius, 2019). Identify medication interactions and follow-up as needed (i.e., additive effects with warfarin [Coumadin]; Burchum & Rosenthal, 2019). |
Rheumatoid Arthritis
Pathophysiology
RA is an autoimmune disorder that prompts the immune system to react by causing inflammation in the synovial membrane; in turn, patients will feel joint stiffness and pain. The synovial membrane is treated as an invader by the immune system. This causes the activation of T-cells, the release of cytokines (including tumor necrosis factor [TNF] and interleukin-1), and the formation of antibodies. Genetically, individuals at greater risk of developing RA will express the human leukocyte antigen (HLA)-DRB1 gene, which can impact the immune response related to synovial inflammation. RA causes loss of the synovial fluid and calcification of the joint; over time, this causes a decrease in the range of motion. RA also has systemic bodily effects; it can damage the heart and vasculature, increasing the risk for myocardial infarctions (MIs) and cerebral vascular accidents (CVAs). In up to 80% of individuals, laboratory testing will confirm the presence of rheumatoid factor (RF) and autoantibodies in the serum (Hoffman & Sullivan, 2020; Norris, 2019; Scanlon & Sanders, 2019).
Locally, the autoimmune response includes the activation of neutrophils, macrophages, and lymphocytes. The neutrophils and macrophages cause lysosomal enzymes to be released, which destroys the joint cartilage. The inflammation continues cyclically, eventually impacting the synovial cells and subsynovial tissues, causing hyperplasia and vasodilation. The vasodilation contributes to the redness and warmth and promotes increased capillary permeability causing edema. New vasculature is created and worsens inflammation by developing a pannus. The pannus, which is synovial tissue proliferation, destroys cartilage and bone; this contributes to the decreased range of motion and joint instability. Although patients may experience periods of exacerbation and remission, RA is a progressive and irreversible disease (Cajas et al., 2019; Norris, 2019).
Risk Factors/Protective Features
Currently, 1.5 million Americans have RA (Arthritis Foundation, 2021). Approximately 1% of individuals are diagnosed with RA (Hoffman & Sullivan, 2020). Genetics related to joint inflammation and over-activation of the immune system is correlated with a diagnosis of RA. Individuals with Pinna or Chippewa Indian heritage are at higher risk for developing RA. Adults in their 6th decade are at the greatest risk for developing RA; women are at a 2 to 3 times greater risk for developing RA than men. Lifestyle factors, such as obesity and smoking, can increase an individual's risk of developing RA. Additional risks include having not given birth before, with the presence of HLA class II genotypes, and childhood exposure to second-hand smoke. Other environmental causative factors include exposure to viruses and bacteria in the gut. Patients may enter remission within a year of diagnosis; if not, they are more likely to experience disability within 10 years (Hinkle & Cheever, 2018; Hoffman & Sullivan, 2020; Norris, 2019).
Signs and Symptoms
While OA may have unilateral joint involvement, individuals with RA have bilateral joint involvement and disability. Joint stiffness can last 30 minutes to several hours; pain that prevents movement can later contribute to fibrosis (Hoffman & Sullivan, 2020). The most common sites of RA include the hands, wrists, knees, and ankles (Arthritis Foundation, 2021; CDC, 2020b; Norris, 2019). RA affects the smaller joints in the fingers, wrists, and ankles first (Arthritis Foundation, 2021). The hands may become deformed, and patients may exhibit swan-neck deformities and ulnar deviation due to edema, joint destruction, or subluxation (see Figure 3). In addition, patients may experience Heberden’s and Bouchard’s nodes in the fingers. Heberden’s nodes are swollen, hard, and painful distal interphalangeal (DIP) joints of the fingers. Bouchard’s nodes are similar but occur at the proximal interphalangeal (PIP) joint (see Figure 4). Over time, the cervical spine may become impacted. When the cervical spine is diseased with RA, patients may describe neck discomfort, occipital headaches, and muscle weakness in the upper arms (Hinkle & Cheever, 2018; Norris, 2019).
Figure 3
Swan-Neck Deformity
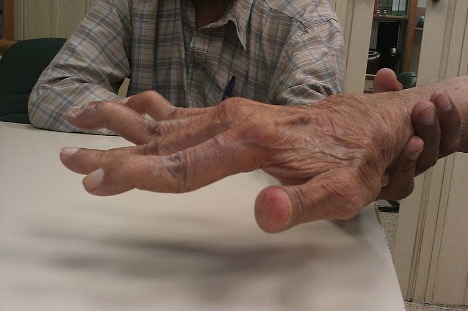
(Phoenix119, 2014)
Figure 4
Heberden’s and Bouchard’s nodes

(Lengerke, 2009)
RA has systemic involvement; thus, systemic signs and symptoms are likely present. Patients may experience fatigue, anorexia, weight loss, generalized aching, and stiffness (Hoffman & Sullivan, 2020). RA has been noted to affect the heart, lungs, and eyes. Patients may report a low-grade fever, dry mouth, gum inflammation, shortness of breath, dry eyes, and photosensitivity. The heart and blood vessels may be inflamed, and serum laboratory testing may indicate anemia. The patient may have rheumatoid nodules, which are small lumps surrounding small blood vessels located on or near bony prominences (Arthritis Foundation, 2021; Norris, 2019).
Diagnosis
A diagnosis of RA is confirmed using a physical assessment, serum laboratory tests, and imaging (CDC, 2020b). A history and physical evaluation should be performed to assess for pain, disability, joint tenderness, edema, and temperature changes. The joint may have a spongy-like feel due to inflammation and synovial thickening. The serum laboratory tests will include the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and RF. An elevated ESR and CRP indicate the presence of inflammation. The presence of RF confirms that the patient has RA; however, RF is absent in 20% of individuals with RA. If the laboratory findings demonstrate a high RF, it is likely the patient is experiencing systemic effects of RA. The patient may also have their complete blood count (CBC) checked. Results may show thrombocytosis and anemia due to systemic inflammation (Arthritis Foundation, 2021; Hinkle & Cheever, 2018; Norris, 2019).
Imaging tests include x-ray, ultrasound, or magnetic resonance imaging (MRI) to look for erosions in the bone; bone erosions are a late manifestation of RA. Imaging tests are utilized to assess the effectiveness of RA treatment as well. Synovial fluid may be examined for markers of inflammation; if inflammation is present, the synovial fluid will appear cloudy or yellow (Arthritis Foundation, 2021; Hinkle & Cheever, 2018; Norris, 2019).
Treatment/Management
Nonpharmacological
Patients with RA should be educated on the benefits of rest, physical exercises, and proper body mechanics (Norris, 2019). Patients should also be encouraged to partake in smoking cessation and maintain a healthy weight (CDC, 2020b). Heat and cold therapy have been found to help manage RA (Arthritis Foundation, 2021). Surgery is a last resort; patients may require a synovectomy, tenosynovectomy, arthrodesis, or arthroplasty (Norris, 2019).
Pharmacological
When treating RA, the goals are to enhance the patient’s mobility by decreasing inflammation and slowing the progression of any related disability (CDC, 2020b; Scanlon & Sanders, 2019). Early medication initiation is desired due to the potential responsiveness of T-cell-dependent pathways when combatting inflammation (Norris, 2019). Drug classes may include disease-modifying antirheumatic drugs (DMARDs), NSAIDs, corticosteroid medications, and biologic drugs to prevent further inflammation and combat any that may be present. DMARDs suppress the immune system by combatting pro-inflammatory mediators such as TNF, B-cells, T-cells, and IL-1 (see Table 2). NSAIDs, such as meloxicam (Vivlodex) and naproxen (Naprosyn), and corticosteroids are given to decrease inflammation and pain; however, it is essential to note that they cannot be used concurrently (Norris, 2019). Biologic drugs have been used more frequently in the last 10 years and are effective in treating RA by stopping the inflammatory cascade. Biologics, such as abatacept (Orencia), are given intravenously by a healthcare provider or patient-administered subcutaneous auto-injector (Bristol-Myers Squibb Company, 2021; Hinkle & Cheever, 2018; Hoffman & Sullivan, 2020).
Table 2
DMARDS
Nonbiologic DMARDs: These medications slow disease progression and/or delay onset. Less associated risk and cost than biologics. | |
Methotrexate (Rheumatrex, MTX) = preferred | Monitor CBC, liver, and kidney function. Teratogenic, contraindicated in pregnancy, or when planning pregnancy – both female and male. Folic acid (<5 mg/week) to decrease GI and hepatic side effects (Uribe, 2018). Available as an auto-injector or oral weekly dose. |
Hydroxychloroquine (Plaquenil) | Typically used in combination with methotrexate (Rheumatrex, MTX). Long-term outcomes are improved by early initiation. The full effect may take 3 to 6 months. Retinal damage resulting in blindness can occur. This is dosage-related and can occur after the medication is stopped. An ophthalmologic exam should be completed before treatment and then every six months. Educate the patient to report visual changes immediately. |
Sulfasalazine (Azulfidine) | Has the specific benefit of slowing joint deterioration. Contraindicated in patients with a sulfa allergy. GI distress can be decreased with divided doses and the use of enteric-coated form. Monitor BMP, liver function, and CBC to detect hepatitis and bone marrow suppression, which can occur but are rare. |
Leflunomide (Arava) | Common adverse effects include rash, reversible alopecia, diarrhea, and respiratory infection. Serious adverse effects include severe hypertension, pancytopenia, hepatotoxicity, and Stevens-Johnson syndrome. Monitor liver function monthly, counsel patients to report symptoms of liver failure; contraindicated in patients with liver disease. Contraindicated in pregnancy. There is a protocol to clear the drug using cholestyramine which must be implemented before a planned pregnancy for both males and females. |
Penicillamine (Depen Titratabs), gold salts, azathioprine (Azasan), cyclosporine (Neoral), minocycline (Minocin), Protein A | Used infrequently as a last resort if other medications are not effective. Have a higher risk of toxicity, thus requiring complete and focused assessment when utilized. |
Biologic DMARDs: These medications interfere with the immunologic response to decrease RA symptoms. A significant associated cost and risk of infection and/or cancer. | |
Tissue Necrosis Factor Inhibitors (TNFIs): Interfere with TNF to prevent the release of chemical and immunologic factors that damage joints in RA (and other inflammatory disorders). The medications are each a unique monoclonal antibody that binds to TNF. | |
Etanercept (Enbrel) | The first-line drug when the use of biologics is indicated. Binds with TNF to prevent it from binding with cell receptors. Injection site symptoms may last several days. Risks for fungal infections, opportunistic infections, hepatitis B reactivation, and particularly TB – requires TB testing before and monitoring during treatment. Avoid administration with a concurrent disease with infection risk (i.e., diabetes). Live virus vaccines are contraindicated. Serious adverse effects include severe allergic reactions, heart failure, cancer (especially lymphoma in younger patients), blood disorders, liver failure, and demyelinating nervous system disorders. Withhold 1 week before surgery |
Adalimumab (Humira) | Only indicated for use in adults. Withhold 2 weeks before surgery. See etanercept (Enbrel) for precautions and adverse effects. |
Golimumab (Simponi) | Only used in combination with methotrexate (Rheumatrex). Injection site and upper respiratory tract infections are more common. Withhold before surgery at the recommendation of the provider. See etanercept (Enbrel) for precautions and adverse effects. |
Infliximab (Remicade) | Administration of antihistamines, acetaminophen, and/or corticosteroids, as well as decreasing the infusion rate, can reduce infusion reaction. Stop infusion if anaphylaxis occurs. Withhold 6 – 8 weeks before surgery. See etanercept (Enbrel) for precautions and adverse effects. |
Certolizumab pegol (Cimzia) | Side effects include upper respiratory tract infections, urinary tract infections, and arthralgia. Withhold 2 weeks before surgery. See etanercept (Enbrel) for precautions and adverse effects. |
Non-TNFI biologics | |
Rituximab (Rituxan) | A monoclonal antibody that reduces B lymphocytes by binding with CD20 on the B cell. Indicated when one or more TNF antagonists have not been effective. Infusion reactions can be severe, with onset in 30 to 120 minutes, including angioedema, cardiogenic shock, MI, and bronchospasm. Antihistamine, acetaminophen (Tylenol), and corticosteroids should be given before administration. Corticosteroids, epinephrine, bronchodilators, and oxygen should be immediately available during administration for adverse events. Risks include hepatitis B or reactivation of John Cunningham (JC) virus in the brain of patients treated for non-Hodgkin’s lymphoma, causing progressive multifocal leukoencephalopathy. |
Abatacept (Orencia) | T-cell activation inhibitor. Increases risk for infections and cancer. Specific infection risks include upper respiratory infection, pneumonia, cellulitis, bronchitis, diverticulitis, and urinary tract infections. Not used in combination with TNF inhibitors or anakinra (Kineret) It may cause a decreased response to all vaccines, and live vaccines are contraindicated. |
Tocilizumab (Actemra) | An IL-6 receptor antagonist administered IV. It can be combined with methotrexate (Trexall) but not with TNF inhibitors, other DMARDs, or other immunosuppressant medications. Adverse effects include severe infections and colon perforation, especially in patients with diverticulitis and liver damage. Monitor for increased cholesterol, decreased platelets, and decreased neutrophil count. Decreases drug levels and effectiveness of oral contraceptives, warfarin (Coumadin), proton pump inhibitors, and HMG-CoA reductase inhibitors used to lower cholesterol. |
Anakinra (Kineret) | Blocks receptors for IL-1. Indicated when one or more non-biologic DMARDs have not been effective. Risk for serious infections and contraindicated if active infection is present. Not to be combined with TNF inhibitors. |
Corticosteroids: anti-inflammatory action decreases symptoms until DMARDs are effective and added during flares (Burchum, 2019) | |
Prednisone (Deltasone) prednisolone (Prelone) | The dosage is meant to control symptoms; if decreasing the medication, a 5 to 7-day taper is typical to prevent adverse effects. Potential side effects include gastric ulceration, osteoporosis, and adrenal suppression. |
(Burchum & Rosenthal, 2019; Singh et al., 2015)
Psoriatic Arthritis
Pathophysiology
Psoriatic arthritis is a chronic disease in which the immune system attacks the integumentary system (including skin and nails), joints, axial skeleton, and cardiovascular system (Stober, 2021; Veale & Fearon, 2018). Cytokines, particularly interleukin-17, fuel the inflammation in psoriatic arthritis. Activation of interleukin-17 (IL-17) is due to mast cells, γδ T cells, αβ T cells, and innate lymphoid cells in lesioned skin and synovial fluid. IL-17 triggers the inflammatory cells in skin and joints, such as neutrophils, fibroblasts, osteoclasts, and endothelial cells, causing further inflammation and bone remodeling (Blauvelt & Chiricozzi, 2018). The resulting damage contributes to pain, joint destruction, and erosions. Patients may experience one of the five subgroups: asymmetric, spondylitis, symmetric, distal interphalangeal, and mutilans (Hinkle & Cheever, 2018).
Risk Factors/Protective Features
Individuals with a genetic predisposition to psoriatic arthritis are at a particularly heightened risk when experiencing significant stress (Stober, 2021). Patients with infections and physical trauma due to T-cell activation are also at a heightened risk for psoriatic arthritis (Norris, 2020). Up to 30% of patients with psoriasis will develop psoriatic arthritis (Ogdie et al., 2020). Many patients are between 30 and 50 at the time of diagnosis; there is no significant difference in gender distribution (Hinkle & Cheever, 2018).
Signs and Symptoms
Patients with psoriatic arthritis are likely to experience systemic inflammation. They are likely to have arthritis in their joints, spondylitis, dactylitis (severe inflammation of an entire digit), enthesitis, a form of psoriasis (plaque psoriasis or psoriasis vulgaris), and nail disease. Patients may experience fatigue, back pain, and sleep alterations. Pain may be located in other sites, such as the Achilles tendon, plantar fascia, or tibial tuberosity, due to inflammation at the entheses. In addition, patients may have other conditions with an autoimmune component, such as irritable bowel disease (IBD), obesity, and metabolic diseases (hypertension, NIDDM). Depression and anxiety may also be present (Hinkle & Cheever, 2018; Norris, 2020; Ogdie, 2020).
Diagnosis
Psoriatic arthritis is diagnosed based on the patient’s physical assessment, serum laboratory findings, and imaging results. Patients may have an elevated serum uric acid level (Norris, 2020). Hyperuricemia is due to the fast skin changes that cause the breakdown of nucleic acid into uric acid. This may make it difficult to determine if the patient has gout or psoriatic arthritis (Norris, 2019). Like RA, the CRP and ESR are elevated in psoriatic arthritis; however, RF is absent, differentiating between the two conditions (Hoffman & Sullivan, 2020). Imaging tests may demonstrate spondylitis or asymmetrical sacroiliitis (Hinkle & Cheever, 2018).
Treatment/Management
Nonpharmacological
The goal of treating psoriatic arthritis is to decrease pain and inflammation and protect the skin and joints. Patients may experience relief from discomfort by participating in PT, occupational therapy (OT), increasing physical activity, weight loss, massage therapy, and smoking cessation. Losing 10% of body weight can significantly reduce inflammation. Patients who desire to swim should be educated on the potential for skin irritation from the chlorine in swimming pools. Patients may try alternative therapies such as acupuncture, heat and cold therapy, and methods to decrease stress. Walking may relieve some pain or discomfort (Arthritis Foundation, 2022b; Norris, 2020; Oggdie et al., 2021).
Pharmacological
Patients with psoriatic arthritis can be prescribed biologics and drugs that impact cytokine function (see Table 2). Cytokine inhibitors can target TNF and IL-23 T-helper-17 cell pathways (Veale & Fearon, 2018). Treatment can also consist of DMARDS (such as methotrexate [Trexall], sulfasalazine [Azulfidine], leflunomide [Arava], and apremilast [Otezla]), a phosphodiesterase-4 inhibitor, and a Janus kinase (JAK)/signal transducer and activator of transcription (STAT) inhibitor (Ogdie et al., 2021). A TNF inhibitor, such as adalimumab (Humira), is only prescribed if other treatments are ineffective. Inhibiting the immune system will lead to fewer skin and joint manifestations of psoriatic arthritis (Norris, 2020).
Axial Spondyloarthritis
Pathophysiology
Axial spondyloarthritis is an inflammatory disease in the axial skeleton that causes extensive pain and disability (Ritchlin & Adamopoulos, 2021). The condition comprises two subsets: ankylosing spondylitis (AS) and non-radiographic axial spondyloarthritis (nr-axSpA). AS does not have clear pathogenesis; however, the presence of mononuclear cells points to immune system dysfunction (Norris, 2019, 2020). The inflammation of the spine, including associated pain, contributes to sacroiliitis (inflammation of the sacroiliac joint), an axial spondyloarthritis symptom. Typically, one or both sacroiliac joints are inflamed and painful (Deodhar, 2020). The specific location of the pain is at the entheses, where the ligaments and tendons attach to the bone (American College of Rheumatology, 2021a).
Risk Factors/Protective Features
Currently, 1.5 million Americans have axial spondyloarthritis. Patients tend to be younger and male, with an average onset of disease at 28. HLA-B27 is a significant genetic precursor to axial spondyloarthritis. In AS, HLA-B27 has been identified in approximately 90% of patients. In addition, patients of Alaskan, Siberian Eskimo, and Scandinavian Lapp heritage have higher levels of HLFA-B27 than other cultures. In the western parts of the US and Canada, Native American tribes are at greater risk of developing axial spondyloarthritis. Patients at an increased risk of developing axial spondyloarthritis tend to have other systemic inflammatory diseases such as psoriasis, inflammatory bowel diseases (Crohn’s disease and ulcerative colitis), enthesitis (inflammation of the entheses), and uveitis (American College of Rheumatology, 2021a; Deodhar, 2020; Norris, 2019, 2020).
Signs and Symptoms
The hallmark patient presentation with axial spondyloarthritis consists of back pain and stiffness for over 3 months (Deodhar, 2020). The back pain tends to occur in the lumbosacral region, buttocks, and hips. Patients with AS report having greater back pain when supine; patients may report prolonged stiffness after rest or sleep. Some patients experience peripheral spondyloarthritis (pain, edema, and stiffness in the extremities). Excessive lumbar lordosis (sway-back) and thoracic kyphosis may be present, particularly as the disease worsens. If the kyphosis is severe, the patient may experience heightened pain levels and balance difficulty contributing to an increased risk of cervical fracture. Patients may also experience fatigue, which can be as debilitating as the pain (American College of Rheumatology, 2021a; Hinkle & Cheever, 2018; Norris, 2019, 2020).
Diagnosis
Diagnosing a patient with axial spondyloarthritis is based upon criteria established by the Assessment of Spondyloarthritis International Society. The criteria are multi-faceted and include physical assessment findings, patient history, genetic testing, serum, and radiologic findings. The patient must experience back pain for over 3 months, be less than 45 years old, and have the presence of HLA-B27 and sacroiliitis. In addition, they may have another form of arthritis, IBD, psoriasis, uveitis, enthesitis, dactylitis, respond to NSAIDs treatment, a family history, and an elevated CRP or ESR (Deodhar, 2020). Sacroiliitis may be present on MRI or x-rays (American College of Rheumatology, 2021a). Radiologic findings may include a rigid spine, constricted thoracic cavity, degeneration of the hips, and the presence of arthritis (Norris, 2020).
Treatment/Management
Nonpharmacological
Patients should be encouraged to participate in fitness programs focusing on back exercises. Swimming has significant benefits as it promotes cardiovascular wellness and muscle tone without stressing joints (Norris, 2020). Patients may benefit from OT and/or PT. When lifestyle changes and medication do not provide relief, patients may require surgical intervention, including a total hip replacement or spinal surgery (American College of Rheumatology, 2021a). In the home, patients may benefit from a bed board, a firm mattress, and sleeping in the supine position without a pillow (Norris, 2020).
Pharmacological
NSAIDs may reduce pain and edema. If NSAIDs are ineffective, patients may be prescribed biologics, such as anti-TNF and anti-IL-17 drugs. Anti-TNF drugs include infliximab (Remicade), etanercept (Enbrel), adalimumab (Humira), certolizumab (Cimzia), and golimumab (Simponi). Secukinumab (Cosentyx) is an IL-17 antagonist administered subcutaneously every 4 weeks in the home following patient education on self-administration (Novartis Pharmaceuticals Corporation, 2022). DMARDs, such as sulfasalazine (Azulfidine), may help prevent and treat inflammation and pain. If joint swelling is localized, patients may benefit from corticosteroid injections into joints or tendons (American College of Rheumatology, 2021a).
Gout
Pathophysiology
With gout, patients have high levels of uric acid and urate crystal deposits in the kidneys and joints; typically, the great toe is affected. Patients with primary gout do not have a known cause; whereas, in secondary gout, the cause of the high uric acid levels is known, such as in leukemia, lymphoma treatment, or chronic renal disease. The high uric acid levels can cause increased production of purines and altered degradation of nucleic acids. Patients taking thiazide diuretics are at risk of gout due to urate reabsorption in the kidneys, which increases serum levels of uric acid. The disease has four phases: asymptomatic hyperuricemia, acute gout arthritis, intercritical gout, and chronic tophaceous gout (Hinkle & Cheever, 2018; Hoffman & Sullivan, 2020; Norris, 2019).
During the asymptomatic hyperuricemia phase, the patient may have high uric acid levels; however, they may not experience gout pain. In an acute gout arthritis attack, the patient will have a single joint experiencing inflammation and pain as a flare-up. The time between gout flare-ups is known as intercritical gout; if a patient is not treated, their flare-ups are more likely to increase in frequency and severity. Tophaceous gout is the final phase when the uric acid crystals in the joints are large and form tophi, which can promote bone erosion and joint damage (Perez-Ruiz, 2021).
More specifically, the high levels of uric acid cause monosodium crystal deposits in a joint, leading to an inflammatory response when macrophages attempt to phagocytize the monosodium crystals. The more distal the joint, the cooler the temperature; uric acid crystals are less soluble in temperatures less than 37°C. As such, inflammation in the peripheral joints is expected. Micro-tophi formation further promotes the release of leukocytes and the complement cascade. The inflammation damages the cartilage and subchondral bone, resulting in pain. As the frequency of these events increases, so does the risk for chronic arthritis and tophi. A typical gout attack lasts several days to weeks. After 10 years, the patient may experience chronic tophaceous gout; during this time, the patient will have more recurrent, extensive, and painful gout attacks (Hinkle & Cheever, 2018; Hoffman & Sullivan, 2020; Norris, 2019).
Risk Factors/Protective Features
Men experience gout more frequently than women. African Americans, Taiwanese, Pacific Islanders, New Zealand Maori, and those living in higher-income nations are at higher risk of developing gout. In addition, patients between the ages of 40 and 60 are at a greater risk of experiencing the clinical manifestations of gout. Approximately 3-6% of men in higher-income nations experience gout. High serum uric acid levels place a patient at risk; hyperuricemia is defined as greater than 7 mg/dL in men and 6 mg/dL in women. More specifically, 1 in 5 with hyperuricemia will experience gout, and the prevalence is increasing. Furthermore, patients with a history of hypertension, diuretic use, chronic renal disease, alcohol use, or a diet high in purines and fructose are at greater risk of developing gout. Patients regularly consuming beer have a greater chance of developing gout than those drinking wine or hard liquor (Clebak et al., 2020; Hinkle & Cheever, 2018; Hoffman & Sullivan, 2020; Norris, 2019, 2020).
Signs and Symptoms
Patients may not experience any signs or symptoms during the asymptomatic hyperuricemia phase of gout. From there, pain may be experienced in the distal joints, such as the wrists, fingers, ankles, and heels. The pain may occur at night, following exercise, and after consuming alcohol, medications, or certain foods. The pain and inflammation coincide. The patient will likely experience erythema and edema at the gout attack site; in addition, the patient may experience tenderness on palpation and sensitivity to touch. Asymptomatic periods lasting months to years may separate gout flare-ups. As the frequency of the attacks increases, the patient will progress into the third (intercritical gout) and fourth phase (chronic tophaceous gout; Hoffman & Sullivan, 2020; Norris, 2019).
Although gout signs and symptoms typically affect the distal joints, the disease has a systemic impact. It correlates with CVD, obesity, metabolic syndrome, hyperlipidemia, alcoholism, and renal insufficiency. Any workup for gout should consider these associated or comorbid conditions (Hoffman & Sullivan, 2020; Norris, 2019).
Diagnosis
Gout should be suspected when serum uric acid levels are elevated. The most definitive diagnostic criteria for gout involve aspiration of the synovial fluid via arthrocentesis to assess for tophaceous deposits. A 24-hour urine collection may be considered to evaluate urate excretion (Clebak et al., 2020; Norris, 2019).
Imaging tests may further assess the impact on the joint(s). Early imaging tests may not demonstrate any changes to the joint; however, erosions and nodules become visible in time (Hoffman & Sullivan, 2020).
Treatment/Management
Nonpharmacological
Lifestyle changes can enhance the management of gout. More specifically, patient education focusing on avoiding or limiting alcohol, maintaining a healthy weight, smoking cessation, and avoiding purine-rich foods will help patients with gout (Norris, 2019). Purine-rich foods include seafood, alcohol, and red meats (Clebak et al., 2020). If the joint can be temporarily placed in a splint, the patient may experience less pain (Hoffman & Sullivan, 2020).
Pharmacological
Pharmacological management focuses on relieving pain and decreasing inflammation during an acute gout flare-up. Patients are encouraged to take NSAIDs, such as ibuprofen (Advil) or indomethacin (Indocin), during flare-ups to reduce pain and inflammation. In addition, patients may be given colchicine (Colcrys) or intra-articular corticosteroids for pain and inflammation (Norris, 2019). Long-term medications may be utilized if a patient experiences two or more gout attacks per year or has chronic renal disease, urolithiasis, tophi, chronic gouty arthritis, and joint damage. Allopurinol (Zyloprim) and febuxostat (Uloric) may be used for gout maintenance treatment; allopurinol (Zyloprim) is preferred as febuxostat (Uloric) has a risk of cardiovascular mortality (Clebak et al., 2020). Allopurinol (Zyloprim) should not be used to treat a gout exacerbation as it will alter the serum uric acid levels too quickly and may worsen the flare-up (Hoffman & Sullivan, 2020).
Juvenile Arthritis
Pathophysiology
Juvenile arthritis consists of juvenile idiopathic arthritis (JIA) and juvenile rheumatoid arthritis. JIA is more common. The cause is unknown; however, it is believed that the immune system attacks and permanently damages the joints. Specifically, TNF-alpha, IL-6, and IL-1 contribute to inflammation in the synovial membrane of the joint (American College of Rheumatology, 2021a; CDC, 2020a; National Institute of Arthritis and Musculoskeletal and Skin Disorders, 2021).
Risk Factors/Protective Features
Arthritis impacts 1 in every 1,000 children (American College of Rheumatology, 2021a). Although no specific risk factors have not been outlined, genetics and the environment may contribute to the activation of the immune system against a child’s joint. Juvenile arthritis impacts both male and female children equally and across all ethnicities. The most significant risk factor is having a family member with chronic arthritis and psoriasis (National Institute of Arthritis and Musculoskeletal and Skin Disorders, 2021).
Signs and Symptoms
Juvenile arthritis affects individuals under the age of 16. These individuals may experience periods of remission and exacerbation (i.e., flare-ups). During a flare-up, patients may experience joint pain, edema, erythema, fever, stiffness, rash, fatigue, decreased appetite, and inflammation in the eye. This may cause limping and difficulty with fine motor functions or completing activities of daily living. Patients may experience changes in their skin; they may experience a rash, red patches, or bumps on the skin. The patient may experience changes in their growth, causing one extremity to be shorter than the other. The symptoms are correlated to the joint impacted by the inflammation. Symptoms lasting longer than 6 weeks indicate that a patient’s arthritis is chronic (American College of Rheumatology, 2021b; CDC, 2020a; National Institute of Arthritis and Musculoskeletal and Skin Disorders, 2021).
Diagnosis
Juvenile arthritis is typically diagnosed through a physical assessment, x-rays, and serum laboratory tests conducted by a rheumatologist. After collecting a family and medical history, a physical assessment is performed, including an integumentary and musculoskeletal assessment, gait evaluation, ophthalmic exam (checking for uveitis), an abdominal assessment, and lymphatic assessment checking for edema (CDC, 2020a).
An elevated ESR and CRP indicate inflammation. In addition, autoantibodies are assessed to determine if antibodies are being made to attack the body’s cells and tissues. An antinuclear antibody (ANA) level may be drawn if there is a concern for uveitis; if positive, the patient should follow up with an ophthalmologist. Anti-cyclic citrullinated peptide (CCP) antibodies and RF may be present if a rheumatic component of the arthritis occurs; JIA does not present with positive RF. HLA-B27 may be drawn if enthesitis-related JIA is suspected. A CBC may be ordered to look for infection or anemia; it is not uncommon for patients with juvenile arthritis to experience anemia. A metabolic panel may be ordered to assess renal and liver function (American College of Rheumatology, 2021a; National Institute of Arthritis and Musculoskeletal and Skin Disorders, 2021).
Imaging tests are common to assess for and rule out other conditions. For instance, an x-ray may be ordered to ensure a fracture has not occurred. With JIA, damage to bones will be visible on the x-ray. An ultrasound can visualize the presence of inflammation and excess fluid. MRI scans look at the bones, cartilage, and joints for signs of inflammation and damage (National Institute of Arthritis and Musculoskeletal and Skin Disorders, 2021).
Treatment/Management
Nonpharmacological
Treating a patient with juvenile arthritis requires a holistic approach. The patient may require PT and OT to promote movement, relieve pain, prevent further injuries, and strengthen muscles. The goal is to encourage patients to actively engage in their activities of daily living to their fullest potential. Patients may require care from a mental health practitioner to ensure that they are psychologically coping with the physical and lifestyle changes that come with a juvenile arthritis diagnosis. Patients should be educated on the benefits of rest and exercise on psychological and physical stress and preventing future pain; swimming is recommended as an activity as it does not stress joints. A splint may help prevent the overuse of the affected joint (National Institute of Arthritis and Musculoskeletal and Skin Diseases, 2021).
Patients and their families should be encouraged to live life as normally as possible; they should be encouraged to participate in social activities. Summer camps and support groups for children with similar conditions can promote further coping and holistic well-being. If the patient requires accommodations for school, the provider may fill out accommodation forms that align with Section 504 of the Rehabilitation Act. The patient and family should be given information on social services to ensure access to care and the acquisition of necessary resources to ensure a quality of life (American College of Rheumatology, 2021a).
Pharmacological
Patients with juvenile arthritis may experience significant symptom management from a sound medication regimen. The patient may take DMARDs, NSAIDs, biologic response modifiers (which will inhibit TNF-alpha, IL-1, or IL-6 from causing inflammation), and corticosteroids (National Institute of Arthritis and Skin Diseases, 2021). The treatment regimen requires corticosteroids to be given first, followed by DMARDs and biologics due to the potential side effects of these medications. The pediatric patient and their family should be educated on the side effects of the medications. DMARDS the patient may be given include methotrexate (Rheumatrex, MTX) and leflunomide (Arava; see table 1). Biologic agents include etanercept (Enbrel), infliximab (Remicade), adalimumab (Humira), abatacept (Orencia), anakinra (Kineret;), canakinumab (Ilaris), tocilizumab (Actemra), and rituximab (Rituxan; American College of Rheumatology, 2021a).
Evidence-Based Nursing Practice/Implications for Nursing
Ultimately, nurses should seek to decrease pain and promote physical movement in a patient with arthritis. Nurses should assess mobility, including gait and fall risk (Hinkle & Cheever, 2018). A lack of mobility may disrupt skin integrity and promote a self-care deficit. If a patient experiences a self-care deficit, potential resources within the case management sector are worthy of exploration. Nurses should educate patients on medication and symptom management. For pediatric patients, it is essential to include both the child and the guardian(s). Many medications, especially DMARDs and biologics, have significant side effects; nurses should ensure that the patient knows the side effects when administering medications. In addition, given how disabling arthritis can be, nurses need to assess patients’ social support. As the patient’s body changes, they are at risk for altered body image and chronic pain (Hinkle & Cheever, 2018; Hoffman & Sullivan, 2020).
Diet
A patient’s diet should be assessed and addressed. Patients should be educated on healthy food and drink choices to prevent additional joint stress and inflammation. Compliance is essential to avoid gout flare-ups (Hinkle & Cheever, 2018).
Laboratory Findings
Nurses should frequently assess and address the serum laboratory data. It is essential to have a baseline set of laboratory values, particularly to examine renal function, as the medications used to treat arthritis can negatively impact the kidneys. Medications such as methotrexate (Rheumatrex, MTX) can negatively impact the liver; thus, monitoring liver enzymes is vital (Hoffman & Sullivan, 2020).
Future Research
Genetic Therapy
Currently, research is being conducted to examine if molecular profiling may enhance treatment for RA. More specifically, research is being conducted to examine what genes contribute to resistance to RA treatment. Understanding this will help the medical community treat RA more effectively (Queen Mary University, 2022).
Besides RA, genetic profiling can impact numerous forms of arthritis. Scientists are seeking to understand which gene malfunctions and causes individuals to develop arthritis. This research will help promote gene repair or replacement when treating arthritis (The University of Washington Orthopaedics and Sports Medicine, 2022). Besides treatment, genetic profiling can potentially promote prevention, earlier diagnoses, and treatment of arthritis (Versus Arthritis, 2020).
References
American College of Rheumatology. (2021a). Juvenile arthritis. https://www.rheumatology.org/I-Am-A/Patient-Caregiver/Diseases-Conditions/Juvenile-Arthritis
American College of Rheumatology. (2021b). Spondyloarthritis. https://www.rheumatology.org/I-Am-A/Patient-Caregiver/Diseases-Conditions/Spondyloarthritis
Arthritis Foundation. (2021). Rheumatoid arthritis. https://www.arthritis.org/diseases/rheumatoid-arthritis
Arthritis Foundation. (2022a). About arthritis. https://www.arthritis.org/about-arthritis
Arthritis Foundation. (2022b). Treatments for psoriatic arthritis. https://www.arthritis.org/health-wellness/treatment/treatment-plan/disease-management/treatment-options-for-psoriatic-arthritis
Blauvelt, A., & Chiricozzi, A. (2018). The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clinical Reviews in Allergy & Immunology, 55. 379-390. https://doi.org/10.1007/s12016-018-8702-3
Burchum, J. & Rosenthal, L. (2019). Lehne’s pharmacology for nursing care (10th ed.). Elsevier.
Bristol Myers Squibb Company. (2021). Orencia side effects. https://www.orencia.com/about-orencia/side-effects-of-orencia
Cajas, L. J., Casallas, A., Medina, Y. F., Quintana, G., & Rondón, F. (2019). Pannus and rheumatoid arthritis: Historic and pathophysiological evolution. Revista Colombiana de Reumatología (English Edition), 26(2). https://doi.org/118-128. 10.1016/j.rcreue.2018.10.005
Centers for Disease Control and Prevention (2020a). Childhood arthritis. https://www.cdc.gov/arthritis/basics/childhood.htm
Centers for Disease Control and Prevention. (2020b). Rheumatoid arthritis. https://www.cdc.gov/arthritis/basics/rheumatoid-arthritis.html
Centers for Disease Control and Prevention. (2022). Arthritis data and statistics. https://www.cdc.gov/arthritis/data_statistics/index.htm
Clebak, K. T., Morrison, A., & Croad, J. R. (2020) Gout: Rapid evidence review. American Family Physician, 102(9), 533-538. https://www.aafp.org/pubs/afp/issues/2020/1101/p533.html
Deodhar, A. A. (2020). Understanding axial spondyloarthritis; A primer for managed care. American Journal of Managed Care, 25(17). https://www.ajmc.com/view/axial-spondyloarthritis-primer-for-managed-care
Goldman, L., & Schafer, A. (2016). Goldman-Cecil Medicine, 2 Vol. Set. https://www.relibrary.com/Resource/Title/1455750174
Hinkle, J. L.. & Cheever, K. H. (2018). Brunner & Suddarth’s textbook of medical-surgical nursing (14th ed). Wolters Kluwer.
Hoffman, J. J., & Sullivan, N. J. (2020). Davis advantage for medical-surgical nursing: Making connections to practice (2nd ed). F. A. Davis.
Ignatavicius, D. (2019). Appendix B: Concept presentation: Mobility. Teaching and learning in a concept-based nursing curriculum (1st ed). pp. 249. Jones & Bartlett Learning. https://www.r2library.com/Resource/Title/1284127362.
Lengerke, J. (2009). Roe-heberden [image]. https://commons.wikimedia.org/wiki/File:Roe-heberden.jpg
Lewis, S. L., Bucher, L., Heitkemper, M. M., & Harding, M. M. (2017). Medical-surgical nursing: Assessment and management of clinical problems (10th ed.). Elsevier.
Mayo Clinic Health Letter. (2018). Thumb Arthritis. www.HealthLetter.MayoClinic.com
National Institute of Arthritis and Musculoskeletal and Skin Disorders. (2021). Juvenile idiopathic arthritis. https://www.niams.nih.gov/health-topics/juvenile-arthritis
Norris, T. (2019). Porth’s pathophysiology: Concepts of altered health states (10th edition). Wolters Kluwer.
Norris, T. (2020). Porth’s essentials of pathophysiology (5th ed). Wolters Kluwer.
Novartis Pharmaceuticals Corporation. (2022). Cosentyx Injection Resources. https://www.cosentyx.com/how-to-inject-cosentyx
Ogdie, A., Coates, L. C.., & Gladman, D. D. (2020). Treatment guidelines in psoriatic arthritis. Rheumatology. 59(1), i37-i46. https://doi.org/10.1093/rheumatology/kez383
Open Stax College (2013a). File:907 synovial joints.jpg [Image]. https://commons.wikimedia.org/wiki/File:907_Synovial_Joints.jpg
Open Stax College (2013b). File:909 types of synovial joints.jpg [Image]. https://commons.wikimedia.org/wiki/File:909_Types_of_Synovial_Joints.jpg
Pechar, J. E. (2020). Osteoarthritis. In Harris, C., Adult-Gerontology Acute Care Practice Guidelines (1st ed. pp. 311-312.). Springer Publishing Company.
Perez-Ruiz, F. (2021). Gout. UpToDate. Retrieved September 1, 2022, from https://www.uptodate.com/contents/gout-beyond-the-basics
Phoenix119. (2014). Swan neck deformity in a 65-year-old rheumatoid arthritis patient- 2014-05-27 01-49.jpg [Image]. https://commons.wikimedia.org/wiki/File:Swan_neck_deformity_in_a_65_year_old_Rheumatoid_Arthritis_patient-_2014-05-27_01-49.jpg
Queen Mary University. (2022). New study shows genes can predict response to arthritis treatment and paves the way for future drug development. ScienceDaily. https://www.sciencedaily.com/releases/2022/05/220519115305.htm
Ritchlin, C., & Adamopoulos, I. E. (2021). Axial spondyloarthritis: New advances in diagnosis and management. British Medical Journal, 372. https://doi.org/10.1136/bmj.m4447
Scanlon, V. C., & Sanders, T. (2019). Essentials of anatomy and physiology (8th ed.). F. A. Davis.
Schub, T., & Schiebel, D. A. (2017). Arthritis, rheumatoid: In older adult https://research.ebscomedical.com/eds/detail?db=nup&an=T703263
Singh, J. A., Saag, K. G., Bridges, S. L., Aki, E. A., Bannuru, R. R., Sullivan, M. C., Vaysbrot, E., McNaughton, C., Osani, M., Shermling, R, H., Curtis, J. R., Furst, D. E., Parks, D., Kavanaugh, A, O’Dell, J., King, C., Leong, A., Matteson, E. L., Schousboe, J.T., … McAlindon, T. (2016) 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care & Research, 68(1), 1-25. https://doi.org/10.1002/acr.22783
Skou, S. T., Pedersen, B. K., Abbott, J. H., Patterson, B., & Barton, C. (2018). Physical activity and exercise therapy benefit more than just symptoms and impairments in people with hip and knee osteoarthritis. Journal of Orthopaedic & Sports Physical Therapy, 48(6), 439–447. https://doi.org/10.2519/jospt.2018.7877
Stober, C. (2021). Pathogenesis of psoriatic arthritis. Best Practice & Research Clinical Rheumatology, 35(2). https://doi.org/10.1016/j.berh.2021.101694
University of Washington Medicine Orthopaedics and Sports Medicine. (2022). Research on arthritis. https://orthop.washington.edu/patient-care/articles/arthritis/research-on-arthritis.html
Uribe, L. M. (2018). Arthritis, rheumatoid: Treatment with methotrexate. https://research.ebscomedical.com/eds/detail?db=nup&an=T703376
Veale, D. J., & Fearon, U. (2018). The pathogenesis of psoriatic arthritis. The Lancet, 391(10136), 2373-2384. https://doi.org/10.1016/S0140-6736(18)30830-4
Versus Arthritis. (2020). Genetics and genomics help to predict and treat arthritis. https://www.versusarthritis.org/news/2020/march/genetics-and-genomics-how-research-is-helping-us-to-predict-and-treat-arthritis/