About this course:
This course aims to ensure that all advanced practice registered nurses (APRNs) within primary care and emergency medicine are aware of and able to implement the most up-to-date information regarding the pathophysiology, signs, symptoms, risk factors, diagnosis, and treatment of asthma in their practice.
Course preview
Asthma (for APRNs)
This course aims to ensure that all advanced practice registered nurses (APRNs) within primary care and emergency medicine are aware of and able to implement the most up-to-date information regarding the pathophysiology, signs, symptoms, risk factors, diagnosis, and treatment of asthma in their practice.
After this course, learners will be prepared to:
- formulate an understanding of the epidemiology and pathophysiology of asthma and its various phenotypes
- identify the various asthma risk factors and methods for prevention
- reference the appropriate tests and evaluations used to diagnose asthma
- discuss the lifestyle changes and environmental adjustments that can be instituted to reduce the symptoms and severity of asthma
- discuss the immunotherapy options for the treatment of asthma in those with known allergies
- explore the various assessment and management guidelines available to guide practice, including the Expert Panel Report (EPR)-4 2020 updates and Global Initiative for Asthma (GINA) guidelines
- summarize nonpharmacological and pharmacological options for the management of asthma
Asthma is a chronic inflammatory airway disorder that causes the narrowing of the airways and is the most common chronic disease diagnosed in children. It is characterized by airway hyperresponsiveness and recurrent episodes of acute symptoms such as wheezing, coughing, chest tightness, and shortness of breath (SOB) which affects approximately 262 million people and causes 455,000 annual deaths worldwide (World Health Organization [WHO], 2023). According to the Centers for Disease Control and Prevention (CDC), asthma affects more than 26 million Americans. It is responsible for over 439,000 hospitalizations, 1.7 million emergency department (ED) visits, and over 10 million office visits to medical providers annually. Asthma costs over $50 billion annually to treat and causes over 13.8 million missed school days and 14 million missed workdays in the US alone. Approximately 10 people die due to asthma every day, with non-Hispanic Black individuals being two to three times more likely to die due to asthma complications than any other racial group (CDC, 2022a).
Amongst patients diagnosed with asthma, 60.1% of adult and 44% of pediatric patients have poorly controlled symptoms. Individuals with poor control report increased disease exacerbation, more significant impact on work or school productivity, increased utilization of healthcare resources, and lower quality of life (QOL). Among pediatric patients, uncontrolled asthma was more prevalent in those designated as boys at birth (49.9%) compared to girls (36.2%). Poor control of asthma symptoms was highest in pediatric patients aged 5 to 11 at 46.6%, followed by those aged 0 to 4 years at 45.8% and those aged 12 to 17 at 40.6%. Uncontrolled asthma is more prevalent in adult females (63.1%) than males (54.7%). Adults aged 55 to 64 have the highest rate of uncontrolled asthma at 65.5%, followed by those aged 35 to 54 at 63.4%, older than 65 at 58.4%, and those aged 18 to 34 at 53.3% (CDC, 2022c, 2022d).
Pathophysiology
Chronic asthma symptoms are related to a combination of inflammation and airway hyperresponsiveness. The presence and severity of asthma are related to a complex interaction between genetic and environmental factors. Some researchers attribute the development of asthma to a combination of atopy (a genetic tendency towards an IgE-mediated overreaction to external triggers), a familial tendency, and exposure to particular childhood upper respiratory infection(s), allergens, or triggers. The hygiene hypothesis suggests that the rise in asthma cases in the US in recent decades is due to Western civilization being overly sanitized, reducing the number of environmental exposures and infections, changing indoor air composition, and thus altering the immune systems of children as they age (Garn et al., 2021; McCance & Huether, 2019).
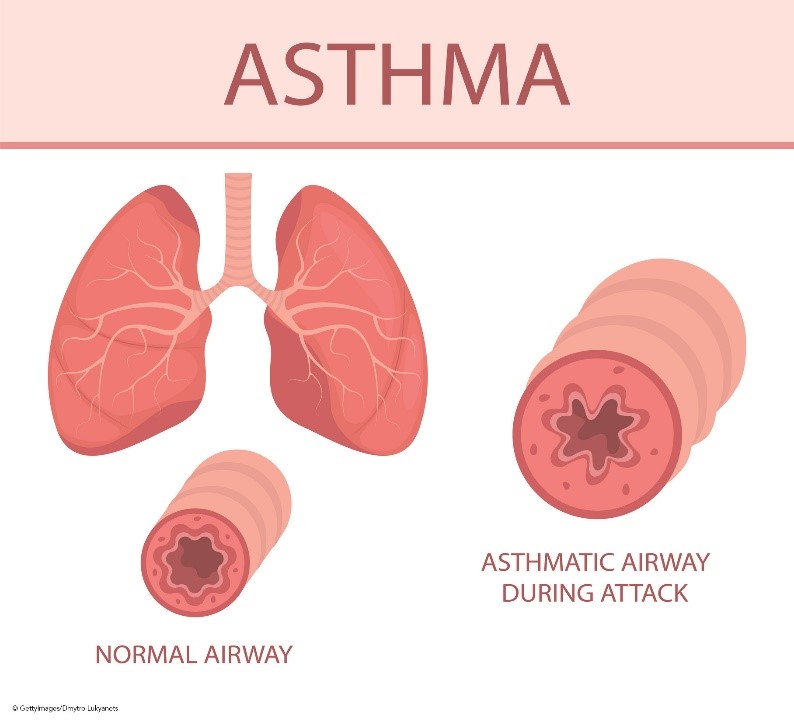
The bronchiolar inflammation and airway constriction lead to resistance and the hallmark symptoms of cough, wheezing, and SOB (see Figure 1). Inflammation may exist without obvious symptoms and can affect the trachea, bronchi, or the smaller bronchioles. The presence of inflammation is attributed to T-helper type 2 (Th2) lymphocytes, eosinophils, mast cells, and neutrophils. These cells form inflammatory infiltrates in the epithelium and smooth muscles of the airway, causing remodeling, including smooth muscle hypertrophy. This hypertrophy leads to airway narrowing and increases the reactivity of the airway to the presence of asthma triggers such as allergens, infections, or other irritants. Airway hyperactivity is also a result of the absence of bronchoconstriction inhibitors such as relaxing factors, prostaglandins, and endopeptidases (enzymes responsible for metabolizing bronchoconstrictors). This lack of inhibitors causes the inflammatory process to persist longer and occur more frequently. In addition, the expansion of mucus-secreting glands and increased mucus-secreting cells cause increased mucus production and decreased mucus clearance, leading to mucus plugs. If left untreated, this chronic inflammation causes damage to the epithelial layer of the respiratory tract, eventually causing permanent fibrotic damage and airway remodeling. Epithelial cells transition to mesenchymal cells, which decreases lung function and responsiveness to treatment. In addition to this underlying inflammation, bronchospasms are caused by sharp contractions of the smooth muscles that line the bronchi (Ortega & Izquierdo, 2022a; Sinyor & Perez, 2022).
Figure 1
Airway Anatomy and Asthma Pathophysiology
Different subtypes of asthma have emerged in the last couple of decades, called phenotypes. The most recent update of the Global Initiative for Asthma (GINA) recognized the existence of these various phenotypes. However, except in patients with severe asthma, they did not feel these phenotypes correlated strongly enough with specific pathological processes or treatment responses to warrant phenotype-specific treatment algorithms (GINA, 2023). According to GINA, some of the most common phenotype classifications include:
- allergic (eosinophilic) asthma
- non-allergic, nonatopic (neutrophilic) asthma
- adult (late) onset
- asthma with persistent airflow limitation
- asthma with obesity (GINA, 2023)
Early-onset atopic asthma, typically diagnosed during childhood, is characterized by reports of wheezing and associated allergies or triggers from external environmental factors such as dust mites, animal fur/dander, and pollen. The pathophysiology of this phenotype is primarily driven by Th2 cells, which then produce the chemical cytokines interleukin 4 (IL-4), interleukin 5 (IL-5), and interleukin 13 (IL-13). IL-4 affects airway epithelium production of chemokines and promotes B-cell isotype switching and T-helper 2 cell development. IL-5 stimulates the activation, migration, and proliferation of eosinophils, releasing toxic neuropeptides, which leads to epithelial injury and scarring. At the same time, IL-13 contributes to airway inflammation and remodeling and hyperresponsiveness via increased mucus production, impaired mucociliary clearance, subepithelial fibrosis, and eotoxin production (a chemokine that attracts eosinophils to areas of inflammation). Subsequent exposures to specific allergens or triggers cause an excessive release of IgE by then-activated B-lymphocytes, leading to the release
...purchase below to continue the course
About 5-10% of asthma patients fall into the nonatopic, late-onset eosinophilic phenotype. This phenotype has a much later onset, few to no allergies, more severe disease, and a poorer prognosis. This phenotype was first described in 1999 by Wenzel and colleagues. Symptoms typically include reports of dyspnea on exertion (DOE) and chronic rhinosinusitis. Further examination and testing typically reveal fixed airflow obstruction and a decreased forced vital capacity (FVC) on lung function testing (LFTs), increased residual volume ("air trapping" or dynamic hyperinflation), and nasal polyposis. The definitive diagnosis for this phenotype is positive eosinophilia finding on a bronchial biopsy or induced sputum sample. However, due to the invasiveness, cost, and difficulty of obtaining those tests, this phenotype may be estimated using clinical presentation, LFT results, and a peripheral blood sample showing positive eosinophilia. Sputum analysis may benefit patients that fit the clinical picture of late-onset eosinophilic asthma. However, difficulty and limited access to capable labs are apparent barriers to this being used widely, as only a limited number of asthma treatment centers have access to induced sputum analysis. However, GINA points out that they have been shown to decrease the risk of exacerbations in adult patients with moderate-severe asthma when used in conjunction with clinical management guidelines. These patients should be managed in or referred to a center experienced in this diagnostic technique. This phenotype is primarily believed to be driven by group 2 innate lymphoid cells (ILC2s), which produce IL-5 and IL-13 when activated. Like T and B lymphocytes, ILC2s are derived from lymphoid progenitor cells but do not express antigen receptors and instead function as an essential component of the innate immune system (Asano et al., 2020; GINA, 2023).
Further differentiation can be made amongst asthma patients based on the presence or absence of eosinophilia, the age of onset (as above), the presence or absence of allergic rhinitis, the resistance to or response to certain medications (such as inhaled corticosteroids [ICS] or leukotriene receptor antagonists [LTRAs]), the presence of chronic obstructive pulmonary disease (COPD), gastroesophageal reflux disease (GERD), obesity, and vitamin D deficiency as comorbidities. These differentiations categorize endotypes, subgroups that describe the underlying pathological process, and characteristics of a particular phenotype. An increased understanding of phenotypes and underlying endotypes has impacted pharmaceutical development. As a result, new drugs are being developed that target specific molecules and disease pathways, leading to more individualized treatment, especially for those patients with severe, drug-resistant asthma (McCance & Huether, 2019).
Risk Factors and Prevention
Risk factors for asthma development include environmental allergens or irritants such as indoor and outdoor air pollution, dust mites, mold, chemicals, fumes, cockroach antigens, and smoke. Children with close family members diagnosed with asthma are also at an increased risk. Asthma is also more prevalent in individuals with additional allergic conditions such as eczema, food allergies, or allergic rhinitis. Individuals born prematurely or with low birth weight are also at an increased risk of developing asthma. Obese adults and children also have a higher prevalence of asthma than those with low or average body weight. It is also thought that vitamin D deficiency may play a role in asthma development and wheezing in children as vitamin D suppresses inflammatory Th17 cells, IgE expression, and Th2-mediated allergic disease and contributes to proper lung development and infection control. Statistics regarding asthma prevalence indicate that it is more common in boys (6.1%) than girls (6.0%); in adulthood, it is more commonly found in women (10.4%) than men (6.2%). It is more common in non-Hispanic Black and American Indian/Alaska Native individuals at 10.8%, followed by non-Hispanic White individuals at 7.6%, Hispanic individuals at 6.7%, and Asian individuals at 3.5%. Asthma rates are also related to increased poverty levels (CDC, 2022b; McCance & Huether, 2019).
Asthma prevention is poorly understood; however, the Global Initiative for Asthma (GINA) makes the following recommendations for asthma prevention. Children should not be exposed to tobacco smoke, including while in utero. There is conflicting data regarding the benefits of vitamin D supplementation in preventing asthma; however, when the results of multiple trials are combined, there was a 25% reduction of asthma and wheezing risk in children aged 0 to 3 but no impact on those children over the age of 6. This benefit was seen with individuals that maintained vitamin D levels of at least 30 ng/mL throughout pregnancy to the time of delivery. One study demonstrated that high-dose fish oil supplementation during the third trimester of pregnancy decreased the prevalence of asthma and wheezing in preschool-aged children; however, the study did not define what constitutes high-dose fish oil or establish a dosing regimen. The use of acetaminophen (Tylenol) and antibiotics during pregnancy and in children is associated with the development of asthma in children and should be avoided. Birth via vaginal delivery may also decrease the risk of asthma, as asthma rates in children born via cesarean section are higher than in those born vaginally (GINA, 2023).
The adverse effects of cooking with indoor gas stoves have been studied in recent years. It is estimated that 35% of US households cook utilizing gas appliances. Researchers analyzed 27 reports focused on the effects of gas cooking on children and found that indoor gas stoves are associated with an increased risk of childhood asthma, accounting for 12.7% of all childhood cases. This risk is comparable to that associated with secondhand tobacco smoke exposure. The risk varied with location, and researchers estimated that Illinois has the highest burden at 21.1%, followed by California at 20.1%, New York at 18.8%, and Massachusetts at 15.4% (Gruenwald et al., 2023).
Diagnosis
Diagnosing asthma can be complicated due to its widely variable presentations, as discussed above. Patients may present with high levels of eosinophilic inflammation and few symptoms, severe symptoms and lower levels of eosinophilia and inflammation, or a classic presentation of eosinophilia and inflammation that seem to correspond positively with the level of symptoms. The clinical signs, symptoms, and features of asthma that providers should be watchful for include a history of recurrent or chronic dry cough, wheezing, difficulty breathing, SOB, or chest tightness. Symptoms are typically worse with exertion or at night, often causing nighttime awakenings. Commonly referred to as triggers, symptoms may also increase in the context of viral upper respiratory infections (e.g., respiratory syncytial virus [RSV] or COVID-19), exposure to environmental or occupational allergens or irritants, changes in the weather (especially the presence of cold, dry air), laughing or crying spells, high-stress, exercise, and the presence of GERD. The Asthma and Allergy Foundation of America (AAFA) also includes smoke and air pollution as potential triggers and lists potential allergens, including dust mites, cockroaches, mold, pet fur/dander, mice, and pollen. Additional triggers for specific patients may include medications (such as aspirin or other nonsteroidal anti-inflammatory drugs [NSAIDs] or non-selective beta-blockers), grasses, flowers, or sulfites (AAFA, 2022; National Heart, Lung, and Blood Institute [NHLBI], 2012, 2022; Ortega & Izquierdo, 2022a). Figure 2 provides more information for patients with asthma regarding possible triggers and how best to avoid them.
Figure 2
Common Asthma Triggers

(NHLBI, 2021)
The most recent GINA report states that diagnosis of asthma should be based on history and symptom patterns, as well as evidence of variable expiratory airflow limitation via spirometry testing (FVC and forced expiratory volume [FEV1]) or peak expiratory flow (PEF or peak flow) measurements with reversibility test. For further details regarding GINA's diagnostic criteria, see Table 1 below (GINA, 2023).
Table 1
GINA Diagnostic Criteria, Age 6+
Diagnostic Feature | Criteria for Diagnosis |
History of variable respiratory symptoms | |
Wheeze, SOB, cough, and chest tightness (descriptions may vary based on age, culture, etc.) |
|
Confirmed variable expiratory airflow limitation | |
Documented excessive variability in lung function (one of the tests below) AND documented expiratory airflow limitation |
|
Positive bronchodilator reversibility test (more likely to be positive if bronchodilator is withheld before the test; short-acting beta agonist (SABA) ≥ 4 hours, twice daily long-acting beta-agonist (LABA) 24 hours, and once daily LABA 36 hours |
|
Excessive variability in twice-daily PEF over 2 weeks |
|
Significant increase in lung function after 4 weeks of inhaled corticosteroid (ICS) treatment |
|
Positive exercise challenge test |
|
Positive bronchial challenge test (usually adults only) |
|
Excessive variation in lung function between visits (less reliable; good specificity with poor sensitivity) |
|
(GINA, 2023)
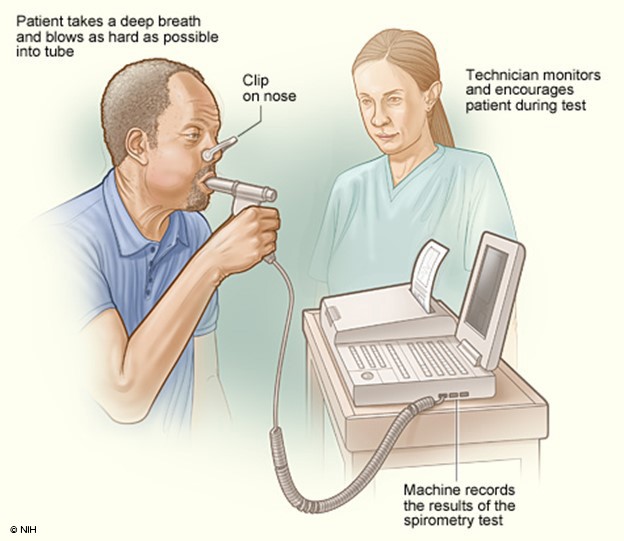
The National Asthma Education and Prevention Program (NAEPP) last published full guidelines, called the Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (EPR-3), in 2007. In 2020, a focused update on six priority topics was released, EPR-4. This report recommends that patients over 5 suspected of having asthma be tested using spirometry before and after a dose of SABA medication (NHLBI, 2020). Spirometry testing (see Figure 3 below) is one of the most commonly used tests to measure pulmonary function. It measures the volume of air exhaled by force at specific intervals following maximal inhalation. It involves having the patient breathe into and out of a mouthpiece connected to a machine or an equipped laptop, with a nose clip to ensure all airflow is directed to the mouth for proper reading. The patient is instructed to take a deep breath and breathe into the mouthpiece as hard and fast as possible. The machine can then produce two primary numbers using these results: the FVC, which is roughly the total lung capacity or volume of air exhaled in liters, and the FEV1, which is the volume exhaled in the first second. A third number is then calculated using these two preliminary numbers, the FEV1/FVC ratio, or the percentage of total lung capacity that the patient can exhale in the first second. These numbers are often compared to the expected capacity/ability based on the patient's size. The patient is tested once, then given a SABA to act as a bronchodilator, and the test is then repeated several minutes later to assess for reversibility. An improvement of 12% or 200mL in the patient's FEV1 or an increase of 10% of predicted FEV1 is typically considered diagnostic for asthma with variable or reversible airflow obstruction; however, an absence of this finding should not exclude trialing treatment with a long-acting bronchodilator (Lamb et al., 2023; Ortega & Izquierdo, 2022a).
Potential barriers to the use of spirometry to diagnose asthma regularly by primary care and pediatric providers include the time required to perform the test, the training needed to administer and then interpret the results correctly, the availability of the necessary equipment, and the misconception that young children cannot correctly perform the test. Spirometry can be especially helpful in ruling out differential diagnoses such as vocal cord dysfunction, functional dyspnea, anatomic obstruction, and restrictive pulmonary defects. There are specific products designed to make spirometry testing easier in children, such as the EasyOne Air, The Micro, and Pneumotrac, to name a few (Stickle, 2018). Spirometry should be repeated at least every year or two in most patients with asthma or more frequently if symptoms are poorly controlled. It should be avoided in patients with either confirmed or suspected infection with COVID-19 (GINA, 2023; NHLBI, 2020).
Figure 3
Spirometry
(NHLBI, 2013b)
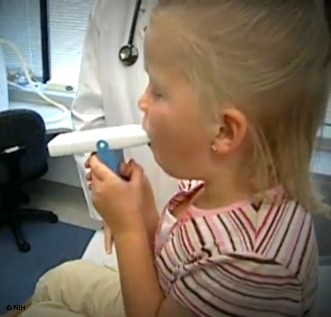
A peak flow meter (see Figure 4 below) is a small handheld device that all patients with asthma should be given for regular monitoring at home. Patients should be instructed to stand, take a deep breath, ensure the indicator is at the bottom of the device, and blow into the device as hard and fast as possible to obtain a score or PEF number. This can be repeated immediately two times, using the highest score out of the three attempts. This should be done at a consistent time, typically first thing in the morning before any medications. Immediately after diagnosis, the patient should do this twice daily (in the morning and around lunchtime) and record the numbers for 2-3 weeks. The highest number of 28 readings from this period will become their personal best (PB). The patient's PB then serves as a benchmark moving forward to indicate a concerning change or drop in lung function. The same procedure may be repeated later during any 2 to 3-week period to reassess and update the 'patient's PB. Their asthma action plan (AAP), a written instruction sheet or road map for patients with asthma to adjust medications and manage their asthma symptoms at home, is based on this PB number. A 'patient's PEF number will often start to decrease before an exacerbation before the patient even identifies any noticeable symptoms, serving as their initial warning sign. A record of highly variable PEF readings and reversibility 10-15 minutes after taking a SABA inhaler may be used as a diagnostic test for asthma if necessary in patients unable to perform spirometry successfully (AAFA, 2017; NHLBI, 2022). GINA recommends that patients also check their PEF following an exacerbation to monitor recovery, following any medication changes, if they feel an increase in their symptoms, and to help identify and pinpoint domestic or occupational triggers. They recommend regular PEF monitoring in patients with a poor perception of airflow limitation, a history of sudden and severe exacerbation(s), or severe or difficult-to-control asthma (GINA, 2023).
Figure 4
Peak Expiratory Flow Meter
(NHLBI, 2013a)
A bronchial challenge test involves attempting to provoke or trigger symptoms by testing spirometry first, then exposing the patient to a chemical irritant such as methacholine (Provocholine) or mannitol (Bronchitol), and then retesting with spirometry. The purpose is to determine if exposure causes a decrease in the patient's FEV1. A bronchial challenge is considered positive if the FEV1 decreases 20% from baseline after exposure to methacholine (Provocholine) or 15% from baseline after exposure to mannitol (Bronchitol; although this may take several exposures of progressively increasing doses). Methacholine (Provocholine) commonly causes wheezing and SOB in patients with asthma, and histamine will cause increased mucus production and bronchoconstriction. Mannitol (Bronchitol), widely used during bronchial challenge testing in other countries, causes airway constriction via mast cell granulation and inflammation. It is approved by the FDA for this purpose but is not widely used in the US (GINA, 2023; Sayeedi & Widrich, 2022; Sverrild et al., 2021).
Fractional exhaled nitric oxide (FeNO) tests the fractional concentration of exhaled nitric oxide. According to GINA, it has not yet been established to rule in or rule out asthma. This is because although FeNO typically increases in patients with asthma characterized by T-helper 2 inflammation but also non-asthma conditions such as eosinophilic bronchitis, atopy, allergic rhinitis, and eczema. It is also not elevated in certain types of asthma, including neutrophilic asthma. FeNO results are often decreased in smokers, during bronchoconstriction, or in the early phases of an allergic response (GINA, 2023).
While preparing the EPR-4 update, the NAEPP conducted a systematic review of the evidence regarding the utility of FeNO in diagnosing and managing asthma. They found that the diagnostic utility of FeNO varies by patient and is more helpful in nonsmokers, pediatrics, and steroid-naïve patients. The sensitivity and specificity varied based on the diagnostic cutoff used. For example, in patients aged 5 and up, if a cutoff score of 20 parts per billion (ppb) or less was used, the sensitivity was 0.79 and specificity was 0.72; if a cutoff score of 40 ppb or greater was used, the sensitivity decreased to 0.41, yet the specificity increased to 0.94. There is insufficient evidence regarding 'FeNO's utility in predicting asthma in patients between 0-4 with chronic wheezing. Regarding the utility during asthma management, the NAEPP found only a weak association between FeNO results and control or risk of asthma exacerbation. However, this association was admittedly stronger in patients with atopy. They found no association between FeNO results and asthma severity. Overall, the results were found to be poorly reproducible. The studies showed that using management algorithms incorporating FeNO results reduced the risk of exacerbations but did not affect the risk of hospitalization, QOL, asthma control, or FEV1. The NAEPP found evidence that FeNO results may help identify patients with asthma more likely to respond to ICS and may help predict impending exacerbations in patients undergoing ICS reduction or withdrawal, but not as a stand-alone test. The clinical context clues improve this predictive ability. Medications can alter FeNO results. Specifically, FeNO results are often lower in patients taking an ICS, an LTRA, or omalizumab (Xolair) but not in patients taking a LABA (Wang et al., 2017).
The role of FeNO in the diagnosis of asthma is still evolving. The updated EPR-4 guidelines recommend the use of FeNO in children over 5 when the diagnosis of asthma is inconclusive after gathering a thorough history, completing a physical examination, and conducting spirometry testing with bronchodilator responsiveness (or in cases where spirometry testing cannot be performed). When FeNO results are elevated (greater than 50 ppb in adolescents and adults or greater than 35 ppb in children ages 5-12), the likelihood that the individual has asthma increases by 2.8 to 7.0 times. However, when reviewing FeNO test results, it is crucial to consider that allergic rhinitis and atopy can increase FeNO levels in patients with or without asthma. The EPR-4 guidelines do not recommend utilizing FeNO in children under 5 as the diagnostic accuracy has not been validated in the literature (NHLBI, 2020).
Assessing for potential differential diagnoses (Table 2) is extremely important when diagnosing asthma, as many respiratory and cardiac conditions often mimic asthma symptoms. For example, COPD in patients over 65 and late-onset asthma may be tough to differentiate, as they will often both respond to certain medications and have similar symptom presentations. Clues that the patient may be developing COPD instead of asthma include a history of smoking or occupational exposure and the pattern of symptom presentation. In contrast, indications that asthma is more likely include nasal polyps or steroid dependency. The most crucial resource in the case of uncertainty regarding diagnosis is a referral to a specialist for confirmation (GINA, 2023).
Table 2
Differential Diagnoses of Asthma with Common Presenting Symptoms by Age
Any Age:
Age 0-5:
Age 6–11:
Age 12–39:
Age 40+:
|
(GINA, 2023)
Nonpharmacological Treatment of Asthma
Asthma management has three components: nonpharmacological treatment, control medications, and reliever medications. The GINA guidelines emphasize a comprehensive list of treatments patients with asthma should be educated about. They recommend smoking cessation (if the patient smokes) or avoiding environmental exposure to secondhand smoke. Patients are also encouraged to engage in regular physical activity, regardless of whether or not the patient has exercise-induced symptoms. To prevent exercise-induced bronchoconstriction (EIB), the patient should be educated on prevention techniques such as completing a warm-up before strenuous activity and administering a SABA, ICS-SABA, or low-dose ICS-formoterol (Symbicort, Dulera) before exercise or wearing a mask or scarf if symptoms are cold-induced. Physical activity is associated with improved symptom control, cardiopulmonary function, and QOL. Another reason physical activity is important is that obesity has been shown to make asthma control more difficult to achieve. Patients who are obese should be strongly encouraged to attempt a comprehensive weight loss plan. All patients with asthma should be educated on the importance of a balanced diet to maintain a healthy weight (GINA, 2023).
Certain medications can cause worsening asthma symptoms, such as non-selective beta-blockers, nonsteroidal anti-inflammatory drugs (NSAIDs), and ACE inhibitors. If cardioselective beta blockers are indicated for treating or preventing coronary events, comorbid asthma is not an absolute contraindication, but the potential benefits versus risks must be weighed. NSAIDs should always be avoided in patients with aspirin-exacerbated asthma, although cyclooxygenase-2 (COX-2) inhibitors and acetaminophen (Tylenol) are usually well-tolerated (GINA, 2023).
Breathing exercises may be used with pharmacological treatment to improve symptoms and QOL. The American Lung Association has helpful patient education videos on their website regarding pursed-lip breathing (which involves breathing in through the nose and then out very slowly through pursed lips, making sure that the exhale lasts at least twice as long as the time spent inhaling), and diaphragmatic breathing (also known as belly breathing, where again the patient breathes in through the nose but places their hands or another object on their belly to reinforce filling the lungs by pulling down and out with the diaphragm into the abdomen, and then slowly exhaling, again taking two to three times the amount of time to exhale as the time spent on inhaling). Although these techniques can be helpful, they have not consistently improved lung function or decreased exacerbations (American Lung Association, 2022; GINA, 2023).
Decreasing stress has been shown to enhance asthma control and lung function beneficially. Patients with asthma should be educated on which stress-relief activity or program fits their lifestyle and personality best. There is insufficient evidence that one technique is more beneficial than another. Anxiety and depression may also lead to worse asthma control, poor adherence, and decreased QOL if not appropriately treated. Patients with symptoms of anxiety or depression affecting asthma control should be referred to a mental health specialist for treatment. GINA also recommends that all patients with asthma pay close attention to their vaccination records and ensure that all recommended vaccinations are current, especially for influenza and COVID-19. Insufficient evidence supports the routine use of pneumococcal and pertussis vaccinations in adults with asthma (GINA, 2023).
The GINA guidelines suggest that patients with asthma avoid exposure to known triggers when possible, especially if these are occupational, indoor allergens, or air pollution. Occupational asthma refers to patients triggered by chemical or environmental exposure during their regular workday, and it generally increases in severity over time. The primary goal of treatment is to limit exposure. Although many patients with asthma may not feel comfortable making drastic decisions such as changing their profession, switching schools, or moving to a rural area with less smog and air pollution, they can at least engage in a program to reduce exposure to indoor allergens in their own homes and schools (GINA, 2023).
An evidence summary conducted in preparation for the EPR-4 found a paucity of high-quality studies looking at the effectiveness of managing indoor allergen exposure and its effect on asthma morbidity and control. Studies that incorporate high-efficiency particulate air filter (HEPA) vacuums appear to decrease the rate of exacerbations and increase the patient's report of QOL. Mattress covers designed to reduce allergens improve non-validated measures of respiratory symptoms when combined with other interventions but have no clinical effect when tested independently. Consistent pest control service reduces the number of asthma exacerbations (Leas et al., 2018). For pediatric patients who spend a large portion of their waking hours in a school environment, a systematic review of multiple randomized control trials was conducted by Maciag and Phipatanakul to evaluate the exposure to environmental allergens and mitigating interventions. Unfortunately, some exposure to allergens is unavoidable, such as pet allergens brought into school on the clothing or belongings of other students. Mouse allergen proteins are also a problem in schools. One study in the review found that 98% of inner-city schools in the northeast US contained detectable amounts of mouse allergen. Exposure to mouse allergen in schools has been associated with an increased likelihood of the individual experiencing an asthma symptom day and a decrease in FEV1 by 4%. The method to decrease mouse allergens is integrated pest management (IPM). Following IPM, studies showed a 75% reduction in mouse allergen correlated to increased lung growth in children. The presence of the cockroach allergens Bla g 1 and Bla g 2 is associated with decreased lung function and increased emergency room visits and hospitalizations in patients with asthma. The most effective intervention to remove cockroach allergens is IPM, which can reduce exposure risk by 90%; however, studies examining the impact of cockroach allergen reduction on asthma symptoms are mixed (Maciag & Phipatanakul, 2022).
The National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC) last published guidelines, called the Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (EPR-3), in 2007. In 2018, the National Heart, Lung, and Blood Advisory Council (NHLBAC) and NAEPPCC established a group to update these guidelines to EPR-4. In 2020, focused updates were released, concentrating on 19 recommendations addressing six topic areas. The NHLBAC also recommended that another 11 topics be acknowledged in the update, but no new recommendations were developed. One such topic was the definitions associated with asthma severity. Due to this lack of update, the original severity classification is still in use, including a practical algorithm for assessing asthma severity at initial diagnosis (see Table 3) based on the frequency of symptoms, nighttime awakenings, SABA use, and exacerbations combined with everyday impairment and spirometry results broken down by age group. Notice that symptoms and lung function testing results vary depending on whether the patient is under the age of 5, 5-11, or over the age of 12, which is typically classified as an adult in most asthma guidelines (NHLBI, 2020).
Table 3
Asthma Severity Classification by Age

(NHLBI, 2012, p. 5)
Once assessed, these severity levels correspond with a stepwise treatment algorithm outlined in the 2020 Asthma Management Guidelines and GINA updates. Step 1 refers to managing intermittent asthma, while steps 2-6 refer to persistent asthma ranging from mild to severe in ascending order. Each step has preferred and alternative treatment options based on patient presentation and preferences (GINA, 2023; NHLBI, 2020).
Immunotherapy
Immunotherapy for treating allergic asthma has become more commonplace and better studied in recent years. GINA guidelines (2023) list this particular therapy among the recommended nonpharmacological treatments that should be considered for patients with asthma. There are two approaches to immunotherapy: subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT). SCIT involves the identification of allergens affecting the individual and then the administration of the extracts of those allergens in progressively higher doses to illicit desensitization. SCIT is associated with decreased symptoms, the need for medication intervention, and airway hyperresponsiveness. The 2020 updates recommend using SCIT as an adjunct therapy conditionally for patients older than 5 with mild to moderate asthma that is well controlled. There are concerns regarding the design of many of the studies conducted on the effectiveness of SLIT. Despite this, SLIT is recommended in patients with asthma and allergic rhinitis related to an established allergy to house dust mites that are suboptimally controlled despite ICS use and have an FEV1 of at least 70% of predicted. SLIT is not recommended in the GINA guidelines or EPR-4 updates to treat allergic asthma specifically (Allergy & Asthma Network, n.d.; GINA, 2023; NHLBI, 2020).
Procedural Treatment Options
Bronchial thermoplasty is a device-based treatment option at step 5 (see Table 4) for adult patients with severe asthma that is uncontrolled despite a regimen of ICS and LABAs and a referral to a specialty asthma treatment center that either do not have access or do not qualify for treatment with biologics. Bronchial thermoplasty is delivered via the FDA-approved Alair system, which uses heat to reduce the amount of smooth muscle in the airways. This decrease in tissue impacts the ability of the airway to constrict, allowing for more airflow and ease of breathing during an asthma attack. GINA guidelines caution against this treatment as studies on the effectiveness are small, and patients did not have their medication regimen optimized before bronchial thermoplasty. The guidelines also cite a significant placebo effect and a transient increase in asthma exacerbations during the 3-month treatment period, followed by a subsequent reduction in exacerbations following treatment. They cited no impact on lung function testing or asthma symptoms compared with sham procedures. GINA guidelines recommend that bronchial thermoplasty only be performed in adults in an Institutional Review Board-approved clinical study until more information is gained on the overall effectiveness of the procedure (Boston Scientific, n.d.; GINA, 2023). Chupp and colleagues completed a study to determine the 5-year efficacy of bronchial thermoplasty on adult patients with severe asthma. A total of 284 subjects between 18 and 65 were included in the study, with 227 subjects completing the entire 5-year follow-up period. The researchers compared the results 5 years post-treatment to the 12 months before treatment. They found that the number of individuals that experienced severe exacerbations decreased from 77.8% to 42.2%, emergency room visits dropped from 29.4% to 7.9%, and hospitalization decreased from 16.1% to 4.8%. The percentage of participants taking oral maintenance corticosteroids also improved from 19.4% to 9.7% (Chupp et al., 2021).
Integration of Care
Asthma is best treated in an integrated, multidisciplinary manner. The School-Based Allergy, Asthma, and Anaphylaxis Management Program (SA3MPRO) is designed by the American Association of Allergy, Asthma, and Immunology (AAAAI) to assist students and their families in managing asthma consistently at home and at school (AAAI, n.d.). It involves four components:
- circle of support- this support and communication network includes the child, the provider, the family, the school registered nurse, and the community
- asthma management plan (AMP)- this includes an AAP (medical authorization for self-carry and administration of asthma medications as needed, parental release of information) in combination with a generic asthma emergency treatment plan (AEP) which is an emergency plan for all students in the school, including stock albuterol and a way to administer the medication
- a comprehensive education plan for all school personnel
- assessment of the school environment with remediation of any triggers present (AAAI, n.d.)
In 2019, with bipartisan support from Congress and other organizations, the AAAAI initiated the School-Based Allergies and Asthma Management Program Act (HR 2468). On January 5, 2021, this Act became law to improve the safety of children with allergies and asthma while in the school setting. The Act requires that the Department of Health and Human Services award grants based on which states ensure that a school nurse or other trained professional is on the school premises and available and that children with allergies or asthma are identified and have an individualized action plan (AAAAI, 2022).
Asthma Action Plan
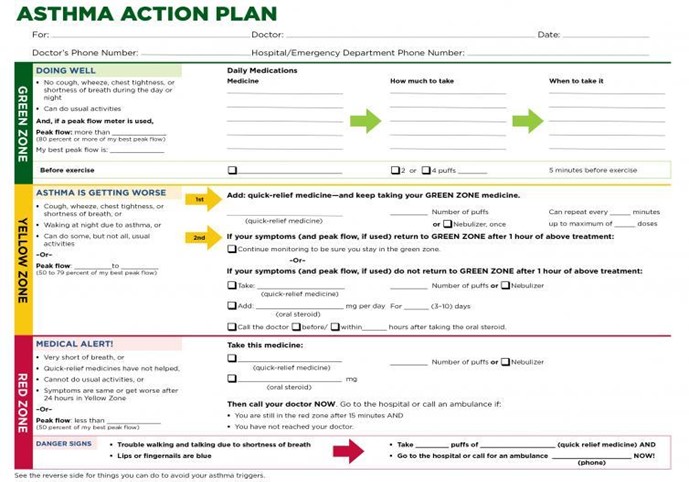
A crucial component of comprehensive care, an AAP, is a 'patient's daily road map for managing symptoms at home. AAPs guide patients on medication changes that can be made in response to symptom or PEF changes or when to seek medical attention. AAPs use a traffic light analogy with green, yellow, and red zones. An AAP helps the patient monitor their symptoms and lung function to identify where they are in the plan (green, yellow, or red zone) and the appropriate actions based on that self-assessment. The green zone is the ideal zone where patients want to be daily. This zone is characterized by the absence of cough, wheeze, chest tightness, or SOB and a peak flow of 80% or more of the individual's best peak flow result. The yellow zone indicates the presence of symptoms such as cough, wheeze, chest tightness, SOB, nocturnal waking due to symptoms, impact on the ability to complete daily activities, and a peak flow of 50% to 79% of the individual's best peak flow result. When in the yellow zone, there is a need for medication administration or adjustment based on the individual action plan. The red zone indicates the presence of an asthma exacerbation or flare-up, usually requiring immediate medical intervention. Symptoms include feeling SOB with difficulty walking and talking, an inability to perform daily activities, signs of cyanosis with discoloration to the lips or fingertips, and a peak flow of less than 50% of the individual's best peak flow result. The red zone also indicates a lack of improvement after administering quick-relief medications or symptoms are the same or worsening after 24 hours in the yellow zone. It is recommended that all patients with asthma, regardless of age, be given a written or electronic AAP that is reviewed and, if needed, edited or updated at each follow-up visit. It is also vital for school-aged children to incorporate the AAP into the school's asthma management program and planning as it outlines the best practices for each student with asthma enrolled (American Lung Association, 2023; GINA, 2023; NHLBI, 2021). See Figure 5 for a sample AAP from the NHLBI.
Figure 5
Sample Asthma Action Plan
(NHLBI, 2021)
Treatment Guidelines
The extensive pharmacological management options available to patients with asthma can overwhelm providers. Options include control medications, typically taken daily on a scheduled basis regardless of symptoms. ICS, LABAs, LTRAs, long-acting muscarinic antagonists (LAMA), theophylline (Theo-24, bronchodilator in pill form), and cromolyn sodium (Intal, a mast cell stabilizer) are control medications. For severe asthma, there are five biologics approved by the FDA. Reliever, quick-relief, or rescue medications work quickly to diminish symptoms, but most have a very short half-life. These include SABAs like albuterol (ProAir, Ventolin) and terbutaline (Bricanyl, Marex; not currently available as an inhaler in the US) or short-acting muscarinic antagonists (SAMA) such as ipratropium bromide (Atrovent). Formoterol fumarate (Symbicort, Dulera) is a longer-acting but rapid-onset LABA also used as a reliever (Sharma et al., 2023).
As previously discussed, the NAEPP last published full guidelines in 2007, with more recent updates in 2020. The original NAEPP guidelines included a practical algorithm to assess asthma severity at initial diagnosis (see Table 3 above) based on the frequency of symptoms, nighttime awakenings, SABA use, and exacerbations in combination with everyday impairment and spirometry results broken down by age group (NHLBI, 2012). Once assessed, these severity levels correspond with the updated EPR stepwise treatment algorithm that assists providers in determining the best medication regimen for their patient, broken down again by age group outlined in Table 4.
Table 4
NAEPP 2020 Asthma Treatment Algorithm
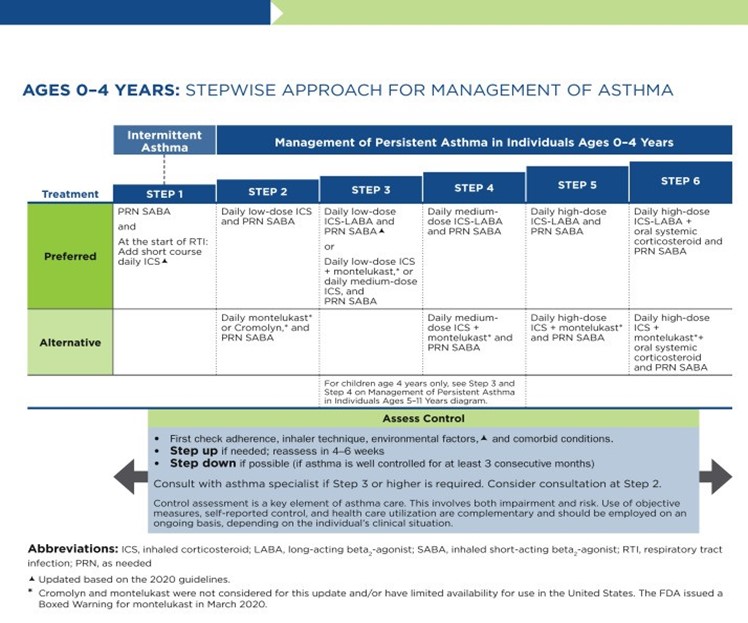
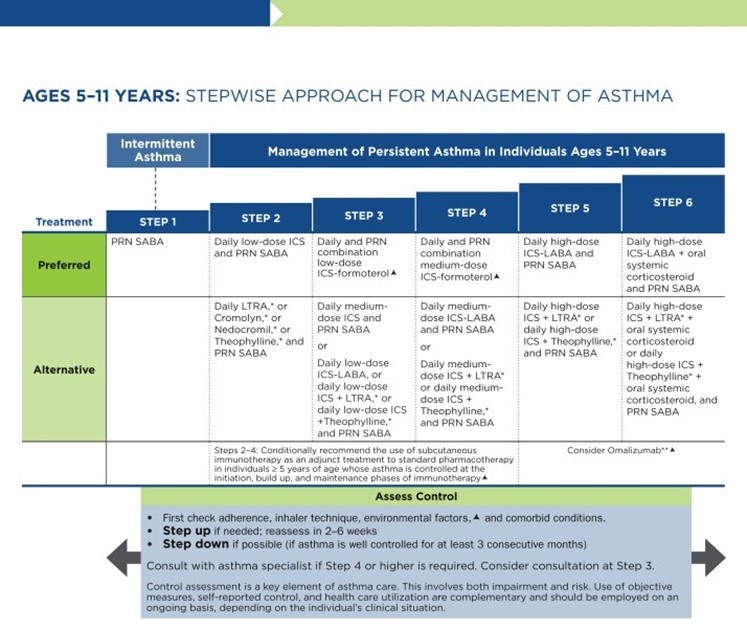
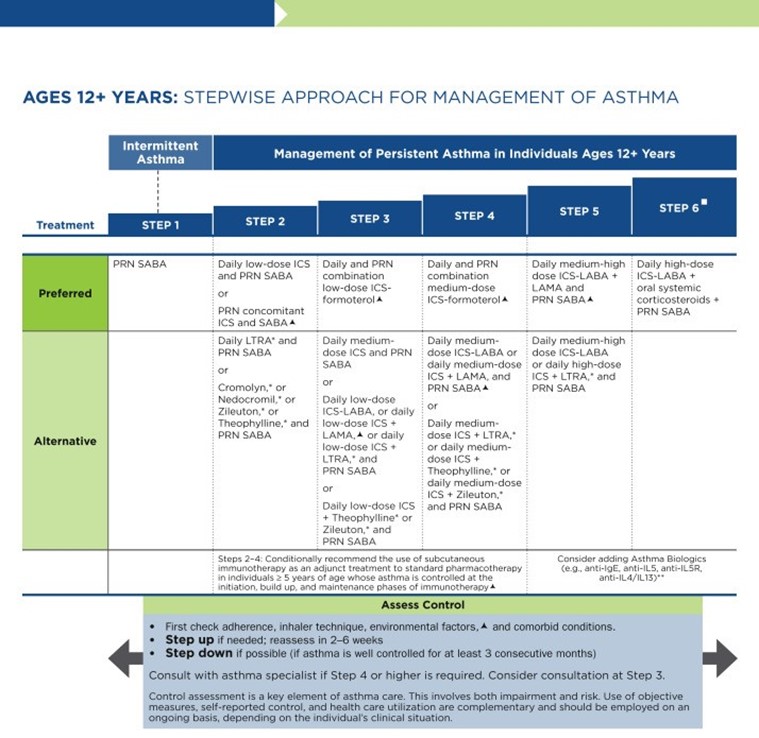
(NHLBI, 2020)
GINA (2023) guidelines also include a stepwise treatment algorithm similar to the EPR-4 2020 updates (see Tables 5 and 6). The primary difference between the two is that GINA's algorithm has just five steps and does not address treatment in children under 6. However, they specify dosage differences based on age (see Table 6 below). Both groups recommend stepping down treatment after 3 months of good control or stepping up treatment for continued symptoms/exacerbations. They both point out the importance of confirming the use of the correct inhaler technique and medication adherence, as well as treating comorbidities such as GERD, obesity, or smoking before stepping up treatment, as all of these conditions may worsen or mask asthma symptoms and lead to inappropriate treatment (GINA, 2023; NHLBI, 2012).
Table 5
GINA Step Guidelines for ages 12+
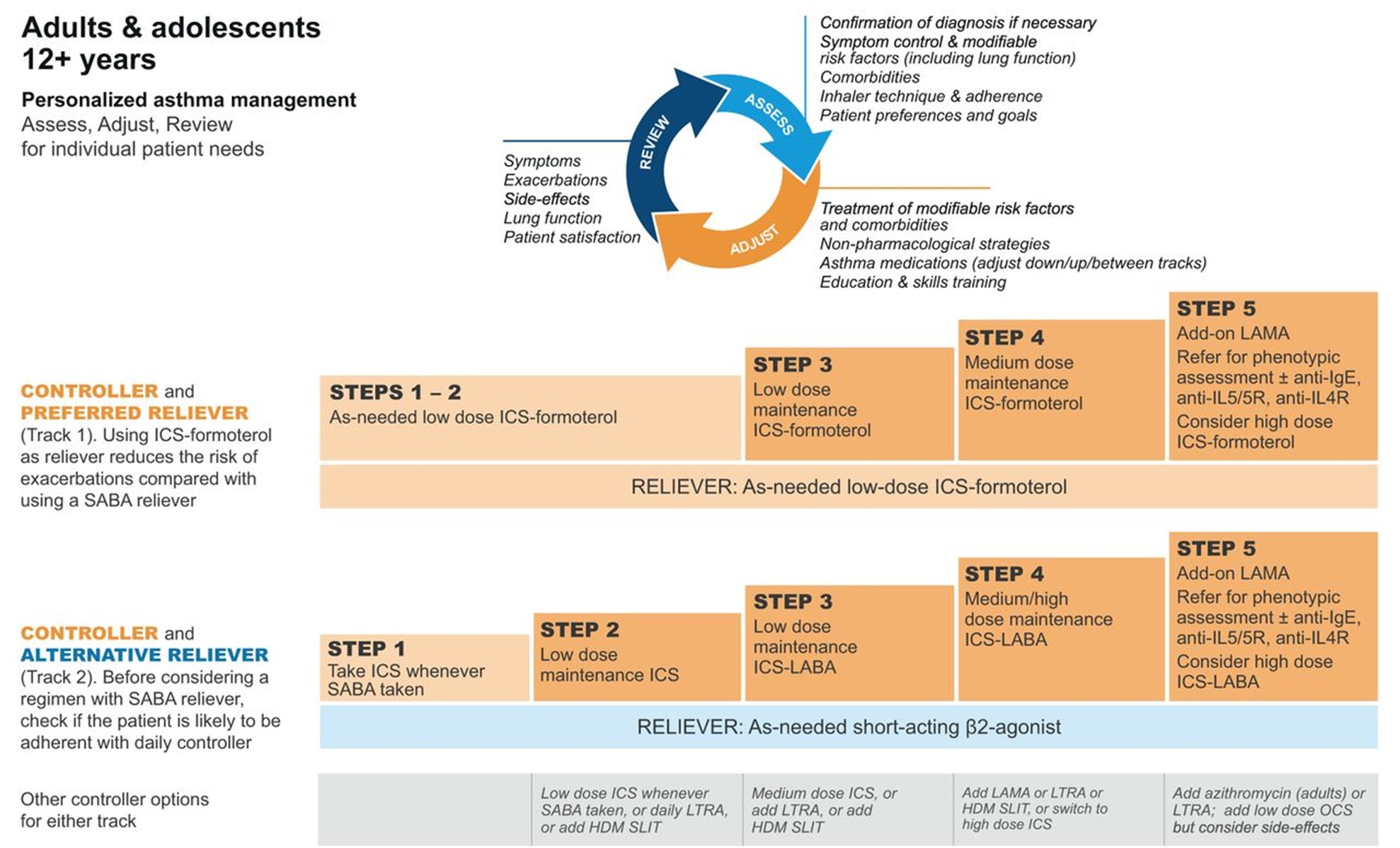
(GINA, 2023)
Table 6
GINA Step Guidelines for ages 6 to 11
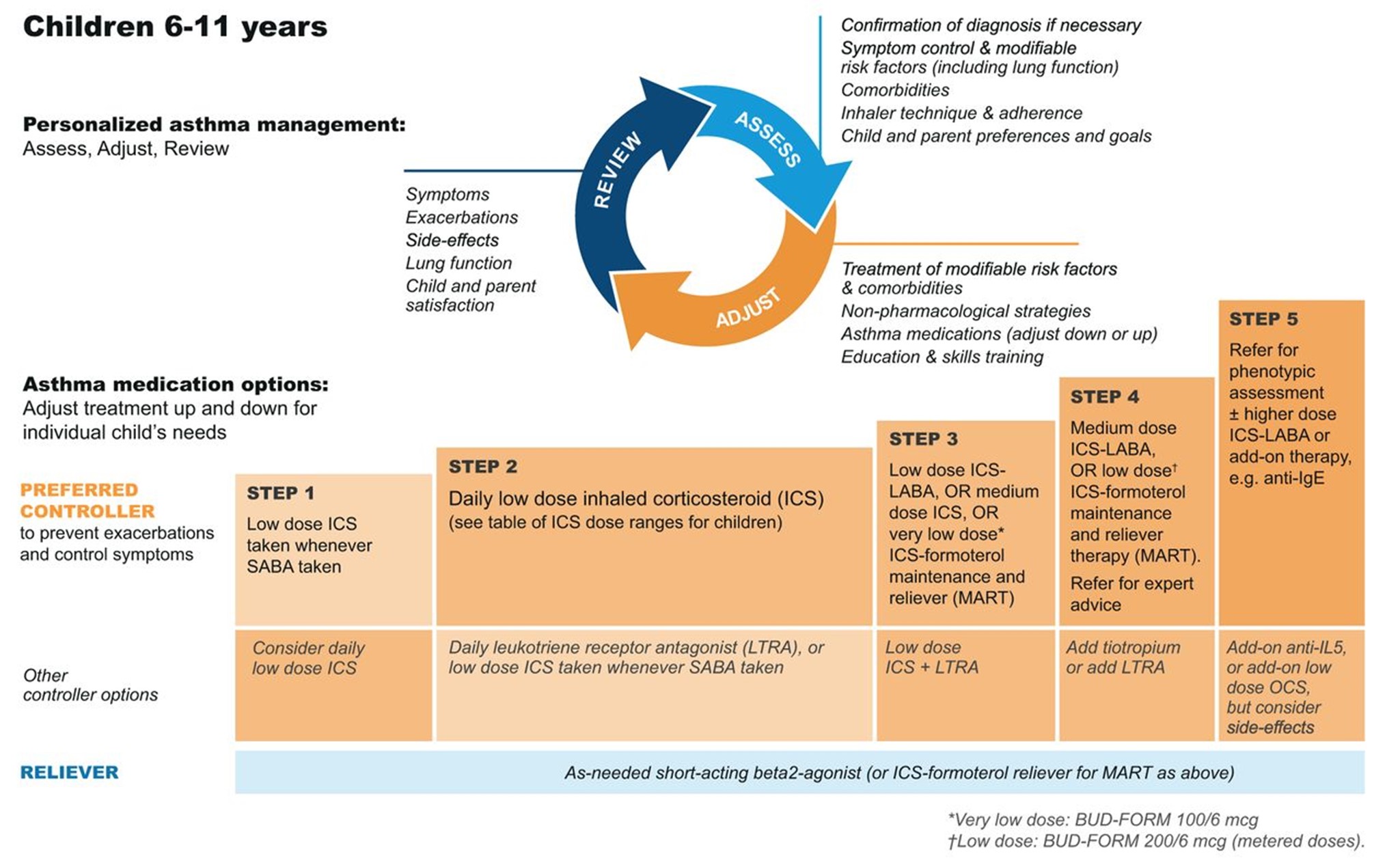
(GINA, 2023)
The first step of the EPR-4 indicates using a SABA as needed for symptom management for all age groups. The severity of symptoms guides treatment intensity, but SABAs may be administered every 20 minutes up to three times. If the administration of intermittent SABA is required more than two days per week, a step up in treatment is indicated (NHLBI, 2020).
Step 2 of the EPR-4 includes preferred and alternative treatments. For all patients, regardless of age, the preferred treatment is the addition of a low-dose ICS administered daily. The alternative treatment for those ages 0-4 is the addition of daily montelukast (Singulair) or Comolyn. The alternative treatment for those ages 5 and older includes the addition of a daily dose of an LTRA, cromolyn sodium (Intal), nedocromil (Tilade), or theophylline (Theo-24). Another alternative option for those ages 12 and older is zileuton (Zyflo; NHLBI, 2020). Step two of the GINA guidelines for children ages 6-11 includes using a daily low-dose ICS. Other controller medications that can be used but are not preferred include daily LTRA or using a low-dose ICS whenever a SABA is used. For those ages 12 and older, the preferred treatment in step 2 is the same as step 1, specifically as needed low-dose ICS-formoterol (Symbicort, Dulera). The alternative therapy is daily low-dose ICS with an as-needed SABA as a reliever medication (GINA, 2023).
Step 3 of the EPR-4 for those ages 0-3 includes increasing the daily dose of ICS to a medium dose. If an escalation to step 4 is indicated, the preferred treatment is the addition of a LABA. The alternative treatment in step 4 includes the addition of montelukast (Singulair). For children that are 4 years old, steps 3 and 4 for those ages 5-11 should be followed. Therefore, step 3 therapy for those ages 4 and up is a low-dose daily and as-needed dose of ICS-formoterol (Symbicort, Dulera). For steps 3 and 4, the preferred quick-relief treatment for children 4-11 is ICS-formoterol (Symbicort, Dulera) 1 to 2 puffs as needed up to a daily maximum of 8 puffs or 36 mcg or a daily maximum of 12 puffs or 54 mcg for those 12 and up. Alternatives for step 3 include a medium dose of ICS with an as-needed SABA, a low dose of ICS-LABA daily, or a low dose of ICS daily plus an LTRA or theophylline (Theo-24) with an as-needed SABA. Step 4 calls for a daily medium dose of ICS-formoterol (Symbicort, Dulera), with an alternative of daily medium-dose ICS with a LABA, LTRA, or theophylline (Theo-24) plus an as-needed SABA. Step 5 treatment is based on daily high-dose ICS-LABA with a PRN SABA, while step 6 involves the addition of a systemic steroid. Alternatives include using an LTRA or theophylline instead of the LABA (NHLBI, 2020).
The primary therapies for Steps 3 and 4 in those over 12 mirror the treatments for 4-11-year-olds, but the alternatives vary some. Alternative step 3 treatments include a daily low dose ICS plus LAMA or zileuton (Zyflo) and a SABA as needed. If a step up is required to step 4 in a patient 12+, the dose of ICS-formoterol (Symbicort, Dulera) increases from a low dose to a medium dose in both the preferred and alternative treatment guidelines. Step 5 treatment is based on daily medium-high-dose ICS-LABA plus LAMA with a PRN SABA, while step 6 involves the addition of a systemic steroid. Alternatives include using an LTRA instead of the LABA (NHLBI, 2020).
Intermittent ICS dosing is defined as varying in dose, frequency, or duration of administration, such as initiating a temporary course of ICS or temporarily increasing the dose. For the 2020 EPR-4 update, an executive summary regarding intermittent ICS and LAMA use was published by Sobieraj and colleagues (2018b), discussing the efficacy of intermittent ICS use by age group. They reviewed 54 randomized controlled trials and two observational studies as part of their process. In patients aged 4 and under with recurrent wheezing, they found intermittent ICS use with SABA (vs. SABA alone) reduced the risk of exacerbation requiring oral steroids based on moderate strength of evidence and improved QOL based on low strength of evidence. In these patients, intermittent ICS reduced the risk of exacerbation requiring oral steroids, hospitalization, or rescue medication use versus regularly scheduled ICS controller use. There was insufficient evidence regarding intermittent ICS use versus nonpharmacological or no therapy in this age group. In patients aged 5 to 11 with persistent asthma, they found intermittent ICS use did not affect QOL or rescue medication use versus ICS controller use based on low strength of evidence, and there was insufficient evidence to assess the effect on the other outcomes in this age group. In patients with persistent asthma aged 12 and older, they found that the use of intermittent ICS dosing, either alone or with ICS controller dosing, versus ICS controller dosing alone, did not affect the risk of exacerbation based on low strength of evidence. However, using intermittent and controller ICS versus controller dosing alone decreased the number of asthma-related outpatient visits based on low strength of evidence (Sobieraj et al., 2018b).
Controller Medications
Inhaled Corticosteroids
ICS are the primary preferred controller medications used in both algorithms for persistent asthma. They reduce bronchial inflammation, prevent exacerbations, and often relieve cough. The onset of action is slow and gradual; therefore, the maximum benefit may take several days to weeks to be seen. There are different standard dosing ranges of ICS based on the age of the patient, the particular guideline followed, and the specific step within the guidelines (GINA, 2023; Liang & Chao, 2022; NHLBI, 2022). The standard low, medium, and high doses of ICS for the GINA and EPR-4 updated guidelines are outlined in Tables 7 and 8.
Table 7
Low, Medium, and High Doses of Inhaled Corticosteroids by Age per GINA
Low dose (mcg/day) | Medium dose (mcg/day) | High dose (mcg/day) | |
Adults/Adolescents (age 12 and older) | |||
Beclometasone dipropionate (Beclovent) MDI, standard particle | 200-500 | >500-1,000 | >1,000 |
Beclomethasone dipropionate (Qvar) dry powdered inhaler (DPI) or MDI, extra-fine particle | 100-200 | >200-400 | >400 |
Budesonide (Pulmicort) DPI or MDI | 200-400 | >400-800 | >800 |
Ciclesonide (Alvesco) MDI | 80-160 | >160-320 | >320 |
Fluticasone furoate (Arnuity Ellipta) DPI | 100 | 200 | |
Fluticasone propionate (Flovent) MDI or DPI | 100-250 | >250-500 | >500 |
Mometasone (Asmanex) MDI | 200-400 | >400 | |
Children (age 6-11) | |||
Beclomethasone dipropionate (Beclovent) MDI, standard particle | 100-200 | >200-400 | >400 |
Beclomethasone dipropionate (Qvar) MDI, extra-fine particle | 50-100 | >100-200 | >200 |
Budesonide (Pulmicort) DPI or MDI | 100-200 | >200-400 | >400 |
Budesonide (Pulmicort) nebules | 250-500 | >500-1,000 | >1,000 |
Ciclesonide (Alvesco) MDI | 80 | >80-160 | >160 |
Fluticasone furoate (Arnuity Ellipta) DPI | 50 | NA | |
Fluticasone propionate (Flovent) MDI or DPI | 50-100 | >100-200 | >200 |
Mometasone furoate (Asmanex) MDI | 100 | 200 | |
(GINA, 2023)
Table 8
Low, Medium, and High Doses of Inhaled Corticosteroids by Age per NHLBI
Medication | Low dose per day | Medium dose per day | High dose per day |
Adults/Adolescents (age 12 and older) | |||
Beclomethasone dipropionate (Beclovent) MDI | 80-240 mcg | >240-480 mcg | >480 mcg |
Budesonide (Pulmicort) DPI | 180-540 mcg | >540-1,080 mcg | >1,080 mcg |
Ciclesonide (Alvesco) MDI | 160-320 mcg | >320-640 mcg | >640 mcg |
Flunisolide (Aerobid) MDI | 320 mcg | >320-640 mcg | >640 mcg |
Fluticasone propionate (Flovent) MDI | 88-264 mcg | >264-440 mcg | >440 mcg |
Fluticasone furoate (Arnuity Ellipta) DPI | 100-300 mcg | >300-500 mcg | >500 mcg |
Mometasone (Asmanex) DPI | 110-220 mcg | >220-400 mcg | >440 mcg |
Children (age 5-11) | |||
Beclomethasone dipropionate (Beclovent) MDI | 80-160 mcg | >160-320 mcg | >320 mcg |
Budesonide (Pulmicort) DPI | 80-360 mcg | >360-720 mcg | >720 mcg |
Budesonide nebules (Pulmicort, Duoresp) | 0.5 mg | 1.0 mg | 2.0 mg |
Ciclesonide (Alvesco) MDI | 80-160 mcg | >160-320 mcg | >320 mcg |
Flunisolide (Aerobid) MDI | 160 mcg | 320-480 mcg | >480 mcg |
Fluticasone propionate (Flovent) MDI | 88-176 mcg | >176-352 mcg | >352 mcg |
Fluticasone furoate (Arnuity Ellipta) DPI | 100-200 mcg | >200-400 mcg | >400 mcg |
Mometasone (Asmanex) DPI | 110 mcg | 220-440 mcg | >440 mcg |
Children (age 0-4) | |||
Budesonide nebules (Pulmicort, Duoresp) | 0.25-0.5 mg | >0.5-1.0 mg | >1.0 mg |
Fluticasone propionate (Flovent) MDI | 176 mcg | >176-352 mcg | >352 mcg |
(Mammen, 2021; NHLBI, 2012)
Since these drugs are administered via inhalation, they are delivered directly to the site of action. Due to this, decreased doses can be used due to the bypass of the first-pass metabolism process. They are traditionally dosed daily or twice daily, regardless of symptoms. Dysphonia is a commonly experienced side effect in 50% to 60% of patients. Dysphonia is reversible after ICS treatment is discontinued. Thrush is also a common side effect of ICS. This is especially true in older adults and those also taking oral steroids. However, the risk of thrush can be reduced significantly with diligent oral hygiene after medication administration and using a spacer or chamber (see Figure 6), which also helps with medication delivery when using a metered-dose inhaler (MDI). In older adults, ICS may also increase the rate of bone mineral loss and cause skin thinning, bruising, and adrenal suppression. Adult patients that have chronically used ICS should undergo bone density measurement. ICS has also been shown to slow growth rates in children by an average of 1 cm, although this is both non-progressive and not entirely predictable. Testing of bone density is not recommended in children; however, it is recommended that children taking ICS intake adequate amounts of calcium and vitamin D (Liang & Chao, 2022; NHLBI, 2022).
Figure 6
MDI with Attached Spacer Device

(FDA, 2012)
GINA guidelines recommend a lower dose of ICS earlier, as opposed to a higher dose, with more significant side effects later. With regular ICS use, an improvement in FEV1 should be seen within days and usually plateaus in roughly 2 months. PEF readings usually increase to PB level after about 2 weeks of treatment, with variability diminishing after 3 months. They also list nasal spray corticosteroids as an alternative for patients with allergic rhinitis and a proven allergy instead of ICS. Patients with eosinophilic asthma typically require a higher dose of ICS for inflammation management, regardless of what may seem like adequate symptom control. Because inflammation can affect the entire respiratory tract, ICS alone may be insufficient in some patients. In severe asthma, systemic steroids may be added as a last resort but should be used cautiously since they carry significantly greater adverse effects (GINA, 2023).
Long-Acting Inhaled Beta 2 Agonists
LABAs are only FDA-approved for use with an ICS (other than formoterol [Perforomist]) and not for acute symptom relief when used to treat asthma. Adding LABA to low-dose ICS in adult patients with asthma who are not well-controlled with low-dose ICS alone results in better control than doubling the ICS dose. However, using LABA alone has been shown to increase the risk of exacerbation and is not recommended. They work as bronchodilators by relaxing the smooth muscles surrounding the airway by selectively stimulating beta-2 adrenergic receptors. Examples of LABAs include salmeterol (Serevent, Advair), vilanterol (Breo Ellipta), and formoterol (Perforomist). Vilanterol's (Breo Ellipta) half-life is 16-21 hours (allowing once-daily dosing) with an onset of about 10 minutes, formoterol's (Perforomist) half-life is 10 hours with an onset of 5 minutes or less, and salmeterol's (Serevent, Advair) half-life is just 5.5 hours with an onset of about 15 minutes. EPR-4 recommends a maximum daily dose of 100 mcg of salmeterol (Serevent, Advair) or 54 mcg of formoterol (Perforomist). They work exceptionally well in patients with a lot of wheezing, SOB, and nocturnal symptoms or those patients found to be in the non-eosinophilic clinical phenotype characterized by severe symptoms but minimal inflammation. Side effects include tremors, vomiting, nausea, fever, headache, cough, dry mouth, and dyspepsia (NHLBI, 2020; Sharma et al., 2023).
Long-Acting Inhaled Muscarinic Antagonists
Tiotropium bromide (Spiriva) is the only LAMA FDA-approved for asthma patients over 11 in the US (although others are approved for treating COPD). It is an antagonist to muscarinic (M3) cholinergic receptors, causing bronchodilation, with a half-life of 25 hours. GINA states that in patients older than 6 with a history of exacerbations not well-controlled on low-dose ICS and LABA, the addition of tiotropium (Spiriva) can be considered in the 4th step. It has been shown to improve lung function and modestly increase the time to severe exacerbation. Dosing is 2 puffs of 1.25 mcg/actuation once daily. It may take 4 to 8 weeks to experience any improvement. The most common side effects experienced are a direct result of the anticholinergic effects of the medication and include urinary retention, dry mouth, headaches, and dizziness. Other side effects include bronchitis, sinusitis, dyspnea, back pain, cough, dyspepsia, and nausea (GINA, 2023; Sharma et al., 2023).
Sobieraj and colleagues (2018b) evaluated the research regarding using LAMA as part of the executive summary when preparing the updated EPR-4. They found that in patients over 11 with uncontrolled persistent asthma, adding LAMA to ICS versus placebo decreased the risk of exacerbation and improved spirometry results. They also compared the combination of LAMA and ICS versus doubling the dose of ICS and found no significant effect. They compared adding LAMA to ICS versus adding LABA and similarly found no significant impact. Finally, they reviewed what they termed "triple treatment," which included ICS, LABA, and LAMA. They found that adding LAMA improved FEV1 based on high strength of evidence and improved asthma control scores based on low to moderate strength of evidence but saw no significant effect on the risk of exacerbation or hospitalization (Sobieraj et al., 2018b). Separately, a systematic review and meta-analysis of LAMA use published in 2018 included 15 randomized clinical trials and over 7,000 patients over 11, including 789 between the ages of 12-17. They found that adding LAMA to ICS versus placebo reduced the risk of exacerbation requiring systemic steroids and improved spirometry results. They found no significant difference between ICS-LABA and ICS-LAMA. Very similar to the results above, when they compared ICS-LABA with triple therapy (ICS/LABA/LAMA), they found an improvement in FEV1 as well as QOL scores but saw no decrease in exacerbation risk (Sobieraj et al., 2018a).
Leukotriene Receptor Antagonists
The EPR-4 and GINA guidelines both list LTRAs such as montelukast (Singulair), zafirlukast (Accolade), or zileuton (Zyflo) as alternative options for persistent asthma treatment in patients aged 5 and above. They are available as once-daily oral tablets, and montelukast (Singulair) is also available in a granule packet. Zileuton (Zyflo) also has a slightly different mechanism of action, working as a 5-lipoxygenase inhibitor to interfere with leukotriene formation, while montelukast (Singulair) and zafirlukast (Accolade) both function as selective LTRAs blocking the leukotriene portion of the inflammatory cascade (Sharma et al., 2023).
GINA specifies that these oral medications may be an appropriate daily controller choice in patients who experience intolerable adverse effects related to ICS or may have concomitant allergic rhinitis and as an alternative in EIB. It is an alternative adjunct to ICS treatment but is less effective than adding LABA. It can also be considered an optional adjunct therapy in patients with aspirin-sensitive asthma (GINA, 2023). EPR-4 specifies that montelukast (Singulair) may be used in children as young as 1, while zafirlukast (Accolade) and zileuton (Zyflo) should not be used in patients under 5. They further specify that both zafirlukast (Accolade) and zileuton (Zyflo) require liver function monitoring regularly. They also suggest LTRAs as an alternative to treating EIB (NHLBI, 2020).
Before prescribing these medications, providers must weigh the benefits versus the risks of adverse effects. Side effects of these medications include headaches, eczema, laryngitis, pharyngitis, dental pain, dyspepsia, cough, sinusitis, hepatitis, and allergic granulomatous angiitis (Churg-Strauss syndrome). Montelukast (Singulair) can cause neuropsychiatric events such as hostility, aggressive behavior, agitation, dream abnormalities, hallucinations, and suicidal behavior, and as of 2020, the FDA has required a black box warning for this serious mental health effect. Due to hepatoxicity risk, LTRAs are contraindicated in patients with hepatic dysfunction (Fanta & Barrett, 2023; Sharma et al., 2023).
Theophylline
Theophylline (Theo-24) is a xanthine derivative administered orally that functions by antagonizing adenosine receptors and increasing cyclic adenosine monophosphate (cAMP), and decreasing the production and release of pro-inflammatory signals, specifically TNF-alpha and leukotriene, thereby causing smooth muscle relaxation and bronchodilation. It can be beneficial with nocturnal symptoms. EPR-4 guidelines recommend theophylline (Theo-24) as an alternative to low-dose ICS in step 2 treatment, an alternative adjunct to ICS or ICS-LABA in steps 3, 4, and 5, or step 6 in conjunction with ICS-LABA in an attempt to avoid a course of oral corticosteroids. It is available in liquid, capsule, or sustained-release tablet form. It is typically dosed at 10 mg/kg per day initially (NHLBI, 2020).
GINA guidelines offer theophylline (Theo-24) as an alternative initial controller medication in adolescents and adults with initial presentation of asthma symptoms, or SABA use more than twice weekly, but note that this is typically less effective than low-dose ICS. They also mention that short-acting theophylline (Theo-24) should not be used as a reliever medication due to its poor efficacy, slower onset of action, and higher risk of adverse effects than SABAs. GINA guidelines also list theophylline (Theo-24) as an adjunct treatment option with ICS or ICS-LABA in adolescents and adults but do not recommend its use in children (GINA, 2023). Potential side effects of theophylline (Theo-24) include headaches, insomnia, irritability, restlessness, seizures, tremors, tachycardia, cardiac arrhythmias, urinary retention, and acute myocardial infarction. Theophylline (Theo-24) may worsen GERD symptoms via lower esophageal sphincter relaxation. Theophylline (Theo-24) also has a narrow therapeutic range and requires serum blood level monitoring at least every 6 months (Sharma et al., 2023).
Cromolyn sodium
Cromolyn sodium (Intal) is a mast cell stabilizer that decreases the degranulation of pro-inflammatory mediators such as histamine and leukotrienes, thereby blocking the cellular response to inhaled antigens that can trigger asthma exacerbations. Cromolyn sodium (Intal) can be administered via MDI or nebulizer; however, a significant limitation of cromolyn sodium (Intal) is needing a nebulizer and needing to be dosed four times daily to be effective. Adverse effects vary based on the route of administration. Effects of inhalation are throat irritation, laryngeal edema, drowsiness, dizziness, bronchial irritation, cough, hoarseness, and cough (Minutello & Gupta, 2023). Cromolyn sodium (Intal) is listed in EPR-4 as an alternative controller medication in step 2; however, insufficient research demonstrates its safety and efficacy in children under 2. It is also one of the options that may be used to pre-treat EIB before exercise (NHLBI, 2020). The updated GINA (2023) guidelines did not mention cromolyn sodium (Intal) or other mast cell stabilizers.
Biologics
For severe asthma, there are six biologics approved by the FDA that work by diminishing or blocking the effects of various steps in the inflammatory cascade, such as omalizumab (Xolair, an antibody that binds IgE), dupilumab (Dupixent, an antibody to IL-4), mepolizumab (Nucala, an antibody to IL-5), benralizumab (Fasenra, an antibody to IL-5), reslizumab (Cinqair, an IL-5 antagonist), and tezepelumab (Tezspire, an anti-thymic stromal lymphopoietin [anti-TSLP]). Omalizumab (Xolair) is the only biologic recommended in the EPR-3 treatment guidelines; when the EPR-4 updates were released, biologic agents were listed as an emerging topic and were not included in the update (NHLBI, 2020). GINA guidelines include all six biologics as an optional adjunct treatment in step 5 for patients over 6 with moderate or severe allergic asthma uncontrolled on step 4 treatment (GINA, 2023).
Omalizumab (Xolair)
Omalizumab (Xolair) is an FDA-approved medication for moderate to severe allergic asthma in patients aged 6 and older with elevated IgE levels. It functions as a monoclonal antibody that binds IgE. Treatment with omalizumab (Xolair) may decrease the frequency of asthma exacerbations, the need for corticosteroids, and symptoms. It is delivered as a subcutaneous injection every 2-4 weeks. The dose is between 75 and 375 mg based on the patient's weight and IgE levels before starting treatment. The manufacturer has a table and tool to calculate dosing based on individual patient values. Adverse effects of omalizumab (Xolair) include headaches, fatigue, peripheral edema, abdominal pain, leg pain, injection site reaction, myocardial infarction, and pulmonary embolism (Ortega & Izquierdo, 2022b; Woods, 2023).
Dupilumab (Dupixent)
Dupilumab (Dupixent) is a monoclonal antibody to the IL-4-alpha subunit receptor that has been shown to significantly reduce exacerbations and improve lung function and asthma control in patients older than 6 with moderate to severe asthma with an eosinophilic phenotype or those that are oral corticosteroid dependent. It is thought to function by inhibiting IL-4 and IL-13 activity. It is given via subcutaneous injection every 2 weeks. There are two dosing schedules. In the first, the initial dose is 400 mg, followed by 200 mg administered every other week. In the second, the initial dose is 600 mg, followed by 300 mg administered every other week. The higher dosing schedule is reserved for patients dependent on oral corticosteroids to reduce the dose or eliminate their use. Side effects include pain or redness at the injection site, sore throat, fever, dizziness, facial rash, diarrhea, toothache, insomnia, eosinophilia, and oral sores (Ortega & Izquierdo, 2022b; Woods, 2023).
Mepolizumab (Nucala)
Mepolizumab (Nucala) is a humanized monoclonal antibody to IL-5 that is FDA-approved for adjunctive therapy for severe eosinophilic asthma in patients 6 and older. Dosing for patients ages 6 to 11 is 40 mg, and for patients 12 and older is 100 mg, administered via subcutaneous injection every 4 weeks. Clinical trials showed that treatment is most effective when serum eosinophil counts are above 0.15 x 109/L; however, this value can be affected by long-term oral corticosteroid use. Mepolizumab (Nucala) has effectively reduced or eliminated the need for treatment with oral corticosteroids. Side effects of treatment include headaches, injection site reactions, abdominal pain, pruritus, eczema, fatigue, and hypersensitivity reactions (GINA, 2023; Ortega & Izquierdo, 2022b; Sharma et al., 2023).
Benralizumab (Fasenra)
Benralizumab (Fasenra) is a humanized monoclonal antibody that binds to the IL-5-alpha receptor, inhibiting eosinophils' proliferation in the bone marrow. It is an FDA-approved adjunctive therapy for patients with severe eosinophilic asthma 12 and older. It has been shown to reduce or eliminate the need for oral corticosteroids and decrease the frequency of exacerbations. The recommended dose is 30 mg administered subcutaneously every 4 weeks for three doses, then every 8 weeks. Potential side effects include headaches, pyrexia, pharyngitis, and hypersensitivity reactions (GINA, 2023; Ortega & Izquierdo, 2022b; Sharma et al., 2023).
Reslizumab (Cinqair)
Reslizumab (Cinqair) is a humanized monoclonal IgG4 antibody to IL-5 that is FDA-approved as adjunctive therapy for patients over 18 with uncontrolled severe asthma with an eosinophilic phenotype. It has been shown to decrease oral corticosteroid use and the frequency of exacerbations. It is administered over 20 to 50 minutes via IV infusion at a 3 mg/kg dose every 4 weeks. Possible side effects include oropharyngeal pain, myalgia, muscle spasms and fatigue, chest and neck pain, elevated creatine phosphokinase (CPK), antibody development, and anaphylaxis (GINA, 2023; Ortega & Izquierdo, 2022b; Sharma et al., 2023).
Tezepelumab (Tezspire)
Tezepelumab (Tezspire) is a novel biologic IgG2 monoclonal antibody that blocks the effects of thymic-stromal lymphopoietin (TSLP). This cytokine is released when the epithelium is irritated by an allergen or another antigen one step upstream from IL-4 and IL-13. It was approved by the FDA in December 2021 for use in patients 12 and older as an adjunct in treating severe uncontrolled asthma. The recommended tezepelumab (Teszpire) dose is 210 mg, administered subcutaneously every 4 weeks. In clinical trials, the most commonly reported adverse effects included pharyngitis, arthralgia, and back pain. There is also a risk for hypersensitivity reactions (AstraZeneca, 2023).
Azithromycin (Zithromax)
For severe asthma, the GINA guidelines recommend adding azithromycin (Zithromax) 500 mg three times weekly after a specialist referral and initiation of high-dose ICS-LABA (Step 5). Before initiating azithromycin (Zithromax), the provider should obtain a sputum sample to test for atypical mycobacteria and an ECG to assess for a prolonged QT interval (this should be repeated after a month of treatment). When utilizing an antibiotic such as azithromycin (Zithromax), the potential for antimicrobial resistance should be considered and weighed against the benefits of treatment. The suggested treatment duration is 6 months, as clinical trials did not demonstrate an improvement after 3 months. The most commonly reported side effect is diarrhea (GINA, 2023).
Quick Reliever Medications
Reliever or rescue medications are used to treat asthma exacerbations or sudden onset of symptoms as needed. Examples of reliever medications include ICS-formoterol (Symbicort, Dulera), ICS-SABA, or SABA alone. Albuterol (Pro-Air, Ventolin) and levalbuterol (Xopenex) are bronchodilators that selectively stimulate beta-2 adrenergic receptors, similar to the previously mentioned LABAs, relaxing the airway smooth muscles. Their half-life is 2.7 to 6 hours. Both are available as MDIs or can be used in nebulizers, and albuterol (Pro-Air, Ventolin) can also be found in oral tablet form, extended-release oral tablet, or liquid syrup. Terbutaline (Bricanyl) is a SABA available in tablet form and a subcutaneous injection with a half-life of 3-4 hours. It is approved for use up to three times daily in patients over 5. EPR-4 and GINA guidelines suggest using SABAs like albuterol (Pro-Air, Ventolin) on an as-needed basis in patients with mild intermittent asthma. As mentioned, formoterol (Perforomist) is a quick-onset LABA with a 10-hour half-life. Ipratropium bromide (Atrovent) is a SAMA that creates bronchodilation by antagonizing acetylcholine receptors. It has a half-life of 2 hours and is available as a nebulizer solution that can be mixed with Albuterol (Pro-Air, Ventolin), levalbuterol (Xopenex), or an MDI that can be used independently. Both guidelines suggest utilizing the 'patient's report of reliever use frequency to help gauge the level of symptom control in patients with asthma (GINA, 2023; NHLBI, 2020; Sharma et al., 2023).
MART/SMART
In the GINA treatment guidelines (2023), low-dose ICS-formoterol (Symbicort, Dulera) is listed in steps 3-5 as an option for controller and reliever medication. This is known as maintenance and reliever therapy (MART) and can only be used with ICS-formoterol inhalers such as budesonide-formoterol (Symbicort, ages 12 to 17). The basic concept of MART is as follows: the traditional instructions to patients with asthma involve one or two inhalers for daily control/maintenance and separate reliever medication in another inhaler to be used as needed. Alternatively, the MART method instructs patients to utilize one inhaler, a combination of ICS and formoterol, a rapid-onset LABA, for maintenance and as-needed symptom control. Some also refer to MART as single-inhaler maintenance and reliever therapy (SMART; the GINA guidelines utilize the term MART and the NAEPP guidelines use the term SMART). MART is also the preferred treatment method in steps 3 and 4 for children ages 6 to 11. Studies have shown that the use of MART with low-dose ICS-formoterol reduces severe exacerbations and provides a similar level of control compared to ICS-LABA or a higher dose of ICS as maintenance medication in combination with a SABA as needed (Evidence level A indicating a large amount of supporting data). One meta-analysis demonstrated that switching patients in Step 3 with poorly controlled asthma to MART led to a reduction of severe exacerbations by 29% compared to an ICS-LABA maintenance dose plus an as-needed SABA (alternative Step 4) and a 30% reduction of symptoms compared to the alternative regimen of Step 3. There is currently no evidence about initiating MART in patients taking biologics; however, there is no contraindication for beginning a biologic in those patients already using MART (GINA, 2023).
The NAEPP also recommends utilizing MART (or SMART) in steps 3 and 4 for those individuals aged 4 to 11 and in step 4 for those older than 12. For individuals 5 to 11, the evidence supports using MART instead of an equivalent, low, or medium dose of ICS-LABA plus an as-needed SABA; however, the evidence was insufficient to make recommendations of MART compared to high-dose ICS-LABA. Three large, randomized control trials (RCTs), including 4,662 individuals older than 12, compared the difference between utilizing MART versus daily ICS (budesonide) plus an as-needed SABA. The results of the RCTs showed a reduction in the relative risk of exacerbations by 35% to 51%. These RCTs recommended the use of MART with a high certainty of evidence. Another analysis of three RCTs by O'Byrne and Colleagues included 224 children ages 4 to 11 who were already being treated with medium to high doses of ICS. Of the 224 participants, 118 were switched to MART, and the other 106 took budesonide (Pulmicort) with an as-needed SABA. In the group that followed MART, the relative risk of exacerbation, including the use of systemic corticosteroids, emergency room visits, hospitalization, and the need for increased medication doses, decreased by 57% compared to the control group. The MART group also demonstrated a significantly improved growth rate of 1 cm due to utilizing a lower dose of ICS (NHLBI, 2020; O'Byrne et al., 2018).
Per the EPR-4 expert panel, more research is needed to address this recommendation. The benefits versus risk of ICS use needs to be differentiated based on race and ethnicity. There also needs to be more research done on the effects on growth for those aged 0 to 4 that require short courses of ICS treatment to treat wheezing triggered by the presence of a respiratory tract infection. The effectiveness of using MART compared to high doses of ICS-LABA with a SABA as needed in children ages 4 to 11. There also needs to be more research done on the effectiveness of other rapid-onset LABAs in combination formulations used for both maintenance and reliever therapy (NHLBI, 2020).
Assessment of Asthma Control
The key to utilizing this stepwise approach effectively is the accurate and consistent assessment of symptom control at each follow-up visit (see Table 9 below). During the follow-up visit, the provider should assess symptom control and frequency of exacerbations since the last visit, lung function, side effects, QOL, and overall patient satisfaction with asthma care. The patient should be asked about nighttime awakenings and daytime symptoms. Medication adherence should be discussed with non-judgmental and open-ended statements and questions. The inhaler technique should be assessed at each visit, and the presence of an AAP that is up-to-date and appropriate should be confirmed. These follow-up visits should occur every 2-6 weeks while the patient is gaining control, every 1-6 months to monitor if well-controlled, or every 3 months if a step-down in therapy is anticipated or being carried out. This follow-up should include spirometry on all patients over the age of 4 at least every year or two, as well as the use of validated symptom control tools in all patients over the age of 11, according to the NAEPP. Good control is a combination of decreased functional impairment and future exacerbation risk or damage (Fanta & Barrett, 2023; NHLBI, 2012).
According to GINA recommendations, follow-up visits to assess control should occur 1-3 months after the initial diagnosis, then every 3-6 months, depending on the level of control. A follow-up is also recommended within a week of an exacerbation requiring an emergency room visit and 3-6 weeks after any medication change. In children that have experienced an exacerbation and are at risk of a repeat exacerbation, a follow-up should be completed within 1-2 days and again in 1-2 months. FEV1 should be reassessed 3-6 months after starting long-term control treatment to establish the patient's PB, and periodically after that, but at least every year or two in asthma patients over the age of 4. GINA guidelines point out persistent bronchodilator reversibility of greater than 12% or 200 mL in a patient on a long-term control medication or who was given a dose of SABA within the last 4 hours or LABA within the previous 24 hours when administered twice daily or 36 hours when administered once daily indicates uncontrolled asthma. Similarly, excessive variability in a patient's PEF readings indicates suboptimal control and an increased risk for exacerbations in the future (GINA, 2023).
Table 9
Follow-up Assessment of Asthma Control

(NHLBI, 2012, p. 6)
An FEV1 less than 60% predicted is an independent predictor for future exacerbation risk and accelerated lung function decrease. This means that if there is a patient with a decreased FEV1 but minimal symptoms, the provider needs to consider whether the patient is significantly limiting their lifestyle to avoid symptom provocation or has a poor perception of airflow limitation secondary to untreated airway inflammation. Conversely, if a patient presents with increased symptoms, but good or acceptable spirometry results, the provider must consider differential diagnoses such as undetected GERD, cardiac disease, or a cough related to post-nasal drip (GINA, 2023).
Asthma control is the extent to which the signs and symptoms of asthma can be observed in a particular patient and how impairments and risk of adverse effects are minimized due to treatment. Control is classified as well-controlled, not well-controlled, and very poorly controlled. The overall goal of asthma treatment is to achieve good control, which is possible in most patients. Patients that have their asthma controlled do not use their quick-reliever medications for more than two days per week, do not experience nighttime awakenings more than twice per month, their PEF or FEV1 is within 20% of their PB, and have not had more than a single exacerbation requiring urgent medical treatment or the use of oral glucocorticoids within the past year (Fanta & Barrett, 2023; Ortega & Izquierdo, 2022a). Asthma control has two facets: symptom control and the risk of future adverse events or exacerbations (GINA, 2023). Numerous screening tools can be used to assess asthma control in adolescents and adults, such as:
- Simple screening tools:
- Consensus-based GINA symptom control tool - a four-question tool that asks about daytime symptoms or use of reliever medication more than twice per week, nighttime awakenings, and any activity limitation in the last 4 weeks
- Primary Care Asthma Control Screening Tool (PACS) - five simple yes/no questions regarding symptoms occurring more than once weekly for the last month
- 30-second Asthma Test- a self-report of symptoms based on five yes/no questions
- Categorial symptom control tools:
- Royal College of Physicians' Three Question Tool - a categorical tool that asks about activity limitation, daytime/daily symptoms, and difficulty sleeping in the last 30 days
- Asthma APGAR tool - includes a patient self-assessment addressing activity limitation, symptom frequency, asthma triggers, adherence to the treatment regimen, and effectiveness of treatment; one US-based study showed that utilizing the Asthma APGAR tool in primary care offices for ages aged 5 to 45 led to improved asthma control, reduced urgent medical treatment (i.e., hospitalizations), and improved adherence to treatment guidelines
- Numerical asthma control tools:
- Asthma Control Questionnaire (ACQ) - a numerical self-assessment of morning symptoms, limitation in activity, nighttime awakenings, SOB, wheezing (in ACQ-5), use of reliever medication (in ACQ-6), and pre-bronchodilator FEV1 (in ACQ-7); final score ranges 0-6, <0.75 is well controlled, 0.75-1.5 yellow/grey zone, >1.5 is poorly controlled; may be used in adults and pediatrics; GINA prefers the use of the ACQ-5 since the question addressing reliever use does not differentiate between regular and as-needed use
- Asthma Control Test (ACT) - a numerical test that includes a patient report of the level of control, limitation in activity, nighttime awakenings, SOB, and use of reliever medication; scores range from 5-25; 20-25 is well-controlled, 16-19 not well controlled, <16 very poor control; childhood ACT (c-ACT) is available with separate sections for the child and guardian to complete
- Other tools designated for use in children include:
- Test for Respiratory and Asthma Control in Kids (TRACK) - for use in pediatrics, includes a recent history of exacerbations
- Composite Asthma Severity Index (CASI) - for use in pediatrics, includes a recent history of exacerbations (GINA, 2023)
Treatment of Exacerbations
The EPR-4 updated guidelines do not address the management of asthma exacerbation. The EPR-3 guidelines (NHLBI, 2012) suggest first assessing the severity of the exacerbation utilizing a physical exam, the patient's report of symptoms, and signs of breathlessness or SOB (audible wheezing, retractions, accessory muscle use). In patients over 5, lung function testing such as spirometry or a peak flow meter may also be used to quantify the limitation in lung function if possible. Supplemental oxygen should be used to treat any existing hypoxemia. Continuous or repeated inhaled SABA (with or without inhaled ipratropium bromide [Atrovent] if severe) should be used to reduce airflow obstruction caused by bronchoconstriction. Ipratropium bromide (Atrovent) is a SAMA that acts as a bronchodilator by antagonizing acetylcholine receptors, similar to tiotropium bromide (Spiriva), a LAMA. Systemic steroids should be started in patients with moderate to severe exacerbation or suboptimal response to SABA treatment to treat the underlying inflammation. The initial assessment should be repeated periodically to assess the response to treatment. Patients who present to the ED for care or are ultimately hospitalized should be discharged with medications (SABA, oral corticosteroids, and possibly the initiation of ICS), a referral for follow-up, an asthma discharge plan, and any patient education that may apply, such as inhaler use and proper technique and environmental trigger exposure (NHLBI, 2012). GINA guidelines list life-threatening symptoms that providers should look for in patients with asthma exacerbations, which include drowsiness, confusion, and a silent chest. They provide the following guidance on admission to the hospital versus discharge home from the ED (GINA, 2023):
- If pre-treatment FEV1 or PEF is less than 25% of predicted or PB, or post-treatment FEV1 or PEF is less than 40% of predicted or PB, hospitalization is recommended.
- If post-treatment lung function is 40–60% of what was predicted, a discharge may be possible after considering the patient's risk factors and availability of follow-up care.
- If post-treatment lung function is greater than 60% of predicted or PB, discharge is recommended after considering risk factors and the availability of follow-up care.
Other factors associated with an increased likelihood of the need for admission include:
- female sex, older age, and non-White race
- use of more than eight beta2-agonist puffs in the previous 24 hours
- the severity of the exacerbation (e.g., need for resuscitation or rapid medical intervention on arrival, respiratory rate above 22 bpm, oxygen saturation less than 95%, final PEF less than 50% of predicted)
- history of severe exacerbations (e.g., intubations, asthma admissions)
- previous unscheduled office and emergency department visits requiring the use of oral corticosteroids (GINA, 2023)
In very severe exacerbations or in those patients who are not responding to the treatments mentioned above, intravenous magnesium sulfate (MgSO4) or helium-oxygen therapy (Heliox) should be considered (NHLBI, 2012). Regarding the use of helium-oxygen therapy (Heliox), GINA guidelines cite a systematic review of studies comparing this to air-oxygen therapy that found no benefit. The guidelines state that they see no role for this treatment in routine care, but it could be considered in patients not responding to conventional treatment; however, cost and availability are barriers to the utilization of this treatment modality (GINA, 2023). The GINA guidelines indicate that randomized trials in patients with mild-moderate asthma showed no benefit from using MgSO4. However, a single 2 gm IV infusion administered to an adult over 20 minutes has been shown to reduce hospitalization rates in:
- adult patients with FEV1 less than 25–30% of predicted at presentation
- patients with persistent hypoxemia who fail to respond to initial treatment
- pediatric patients whose FEV1 falls to less than 60% of what was predicted after 1 hour of treatment (GINA, 2023)
Special Populations
Within asthma care, a few special populations warrant some specific treatment recommendations. Asthma affects 4% to 8% of all pregnancies, making it the most common chronic condition among pregnant individuals. In the US, 8.4% to 8.8% of pregnant individuals have asthma. Uncontrolled asthma during pregnancy can increase the risk of preeclampsia, preterm delivery, low birth weight, or perinatal mortality. Approximately 5.8% of pregnant individuals are hospitalized due to an asthma exacerbation, which is more common during the second trimester. This is due to hormonal and anatomical changes and cessation or reduction of asthma medications solely out of fear of fetal harm. Those with asthma should be monitored closely to ensure the fetus receives enough oxygen. Pregnancy does not always complicate asthma; one-third of individuals do not experience any change, one-third experience improvement, and one-third experience worsening symptoms. An improvement or worsening of symptoms may warrant medication and treatment changes. Although there are concerns regarding administering any medication during pregnancy, the benefits of asthma control greatly outweigh any potential risks (Evidence level A). Most medications used in asthma management are generally acceptable during pregnancy, but they recommend ICS as the preferred long-term controller medication. Unfortunately, there is limited data regarding the use of biologics for severe asthma control during pregnancy; however, it is recommended that pregnant individuals continue taking prescribed biologics, especially if they have been well tolerated and effective at controlling symptoms and decreasing exacerbations. Maintenance and reliever medications should be continued during labor and delivery. Some individuals experience an asthma exacerbation during childbirth due to bronchoconstriction brought on by hyperventilation. In this case, a SABA should be administered. If high doses of SABA are needed during labor, the blood glucose levels of the infant should be monitored for the first 24 hours due to the risk of hypoglycemia, especially if the birth is considered preterm (Chambers et al., 2021; GINA, 2023; Shebl & Chakraborty, 2022).
Approximately 20% of menstruating individuals are affected by perimenstrual asthma (also called catamenial), characterized by worsening asthma control during the premenstrual phase. Risk factors include obesity, severe asthma, and older age. These individuals also tend to have dysmenorrhea, premenstrual syndrome, and shorter cycles but longer periods of active menstruation. In addition to standard asthma treatment, oral contraceptives and LTRAs may be helpful for these patients (GINA, 2023).
Older adults are at a higher risk of medication interactions. All patients should be warned about the risk of osteopenia or osteoporosis with prolonged corticosteroid use, but older patients may be at increased risk for this complication or its sequelae. Older adults also typically see diminishing lung function over time, increased sensitivity to medication adverse effects, and decreased medication clearance. The gradual, progressive decline in spirometry results seen in some asthma patients over their lifetime is fixed airflow limitation. It is usually incomplete and reversible with appropriate treatment. GINA guidelines recommend a streamlined regimen and inhalers that are easy to use (GINA, 2023; NHLBI, 2022).
Adolescent patients will see frequent changes in symptoms, control, and therapy needs due to their rapid growth and hormonal changes during this stage of life. Cognitive and social changes also occur that may influence asthma management. Adolescent patients should be seen separately from their caregivers, at least briefly, to ask about smoking, as smoking rates are higher among adolescents diagnosed with chronic conditions than healthy ones (GINA, 2023).
Those diagnosed with EIB typically experience worsening symptoms following the cessation of physical activity. There is strong evidence that treatment with ICS significantly reduces the incidence of EIB. There is also strong evidence that premedication with a SABA, LABA, or chromones before engaging in physical activity prevents EIB; however, tolerance to SABAs and LABAs has developed with regular (more often than once daily) use (GINA, 2023).
Patients with aspirin-exacerbated asthma typically present with nasal congestion and anosmia, leading to chronic rhinosinusitis with nasal polyps and, eventually, asthma. They usually describe acute exacerbations within 60-120 minutes of exposure to aspirin or other NSAIDs, along with rhinorrhea, nasal obstruction, conjunctival irritation, and flushing of the head and neck. If needed, it can be confirmed with an aspirin challenge test in a well-monitored environment with access to emergency equipment. NSAIDs should be avoided in these patients, but COX-2 inhibitors or acetaminophen (Tylenol) are typically well tolerated. Asthma symptoms can be treated with ICS, LTRA, with or without oral corticosteroids, and desensitization therapy (GINA, 2023).
Referral to a Specialist
Recognizing when a patient needs to be referred to a specialist for more advanced care should be of utmost concern to the provider. In patients aged 4 and under, the EPR-4 suggests referral with an asthma specialist if step 3 management or higher is reached, with consideration at step 2. In patients aged 5 and above, this threshold is step 4 or higher, with consideration in step 3 (NHLBI, 2020). GINA guidelines recommend referral to an asthma specialist at step 5 or in the following circumstances:
- difficulty confirming or doubts regarding the diagnosis
- suspected occupational asthma
- persistent severely uncontrolled asthma or frequent exacerbations despite correct inhaler technique and adherence to treatment
- the presence of any risk factors for asthma-related death, such as ICU admission, mechanical ventilation, anaphylaxis, or a confirmed food allergy
- the risk for or evidence of significant treatment adverse effects (in children, growth delay)
- symptoms that are suggestive of complications or sub-types of asthma, such as aspirin-exacerbated respiratory disease or allergic bronchopulmonary aspergillosis (GINA, 2023)
References
Allergy & Asthma Network. (n.d.). Summary of EPR-4 and GINA asthma guidelines for professionals. Retrieved May 10, 2023, from https://allergyasthmanetwork.org/news/epr-4-vs-gina-asthma-professionals
American Academy of Allergy Asthma & Immunology. (n.d.). The school-based allergy, asthma, and anaphylaxis management program: Comprehensive asthma educational resources. Retrieved May 11, 2023, from https://www.aaaai.org/tools-for-the-public/school-tools/sampro
American Academy of Allergy Asthma & Immunology. (2022). HR 2468, school-based allergies and asthma management program act - approved by Congress. https://www.aaaai.org/About/Advocacy/Legislative-Actions/hr-2468
American Lung Association. (2022). Breathing exercises. https://www.lung.org/lung-health-and-diseases/protecting-your-lungs/breathing-exercises.html
American Lung Association. (2023). Create an asthma action plan. https://www.lung.org/lung-health-diseases/lung-disease-lookup/asthma/managing-asthma/create-an-asthma-action-plan
Asano, K., Ueki, S., Tamari, M., Imoto, Y., Fujieda, S., & Taniguchi, M. (2020). Adult-onset eosinophilic airway disease. European Journal of Allergy and Clinical Immunology, 75(12), 3087-3099. https://doi.org/10.1111/all.14620
Asthma and Allergy Foundation of America. (2017). Lung function tests. https://www.aafa.org/lung-function-tests-diagnose-asthma
Asthma and Allergy Foundation of America. (2022). Allergens and allergic asthma. https://www.aafa.org/allergic-asthma
AstraZeneca. (2023). Highlights of prescribing data: Tezspire (tezepelumab-ekko). https://den8dhaj6zs0e.cloudfront.net/50fd68b9-106b-4550-b5d0-12b045f8b184/e306dc06-d580-4457-b15f-9f28545ad63a/e306dc06-d580-4457-b15f-9f28545ad63a_viewable_rendition__v.pdf
Boston Scientific. (n.d.). The Alair bronchial thermoplasty system. Retrieved May 10, 2023, from https://www.bostonscientific.com/en-US/products/bronchial-thermoplasty/alair-system.html
Centers for Disease Control and Prevention. (2022a). Control asthma. https://www.cdc.gov/sixeighteen/asthma/index.htm
Centers for Disease Control and Prevention. (2022b). Most recent national asthma data. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm
Centers for Disease Control and Prevention. (2022c). Uncontrolled asthma among adults, 2019. https://www.cdc.gov/asthma/asthma_stats/uncontrolled-asthma-adults-2019.htm
Centers for Disease Control and Prevention. (2022d). Uncontrolled asthma among children, 2018-2020. https://www.cdc.gov/asthma/asthma_stats/uncontrolled-asthma-children-2018-2020.htm
Chambers, C. D., Krishnan, J. A., Alba, L., Albano, J. D., Bryant, A. S., Carver, M., Cohen, L. S., Gorodetsky, E., Hernandez-Diaz, S., Honein, M. A., Jones, B. L., Murray, R. K., Namazy, J. A., Sahin, L., Spong, C. Y., Vasisht, K. P., Watt, K., Wurst, K. E., Yao, L., & Schatz, M. (2021). The safety of asthma medications during pregnancy and lactation: Clinical management and research priorities. Journal of Allergy and Clinical Immunology, 147(6), 2009-2020. https://doi.org/10.1016/j.jaci.2021.02.037
Chupp, G., Kline, J. N., Khatri, S. B., McEvoy, C., Silvestri, G. A., Shifren, A., Castro, M., Bansal, S., McClelland, M., Dransfield, M., Trevor, J., Kahlstrom, N., Simoff, M., Wahidi, M. M., Lamb, C. R., Ferguson, J. S., Haas, A., Hogarth, D. K., Tejedor, R… Laviolette, M. (2021). Bronchial thermoplasty in patients with severe asthma at 5 years. Chest Journal, 161(3), 614-628. https://doi.org/10.1016/j.chest.2021.10.044
Fanta, C. H., & Barrett, N. A. (2023). An overview of asthma management. UpToDate. Retrieved May 9, 2023, from https://www.uptodate.com/contents/an-overview-of-asthma-management
Garn, H., Potaczek, D. P., & Pfefferle, P. E. (2021). The hygiene hypothesis and new perspectives - Current challenges meeting an old postulate. Frontiers in Immunology, 12, 637087. https://doi.org/10.3389/fimmu.2021.637087
Global Initiative for Asthma. (2023). Global strategy for asthma management and prevention. https://ginasthma.org/wp-content/uploads/2023/05/GINA-2023-Full-Report-2023-WMS.pdf
Guenwald, T., Seals, B. A., Knibbs, L. D., & Hosgood, H. D. III (2023). Population attributable fraction of gas stoves and childhood asthma in the United States. International Journal of Environment Research and Public Health, 20(1), 75. https://doi.org/10.3390/ijerph20010075
Lamb, K., Theodore, D., & Bhutta, B. S. (2023). Spirometry. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK560526
Leas, B. F., D’Anci, K. E., Apter, A. J., Bryant-Stephens, T., Schoelles, K., & Umscheid, C. A. (2018). Effectiveness of indoor allergen reduction in asthma management: A systematic review. Journal of Allergy and Clinical Immunology, 141(5), 1854-1869. https://doi.org/1016/j/jaci.2018.02.001
Liang, T. Z., & Chao, J. H. (2022). Inhaled corticosteroids. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK470556
Maciag, M. C., & Phipatanakul, W. (2022). Update on indoor allergens and their impact on pediatric asthma. Annals of Allergy, Asthma & Immunology, 128(6), 652-658. https://doi.org/10.1016/j.anai.2022.02.009
Mammen, J. R. (2021). Focused summary (2021) of updated guidelines for asthma management of adults and children ages 12+. https://digitalcommons.uri.edu/cgi/viewcontent.cgi?article=1000&context=oer-healthcare-asthma
McCance, K. L., & Huether, S. E. (2019). Pathophysiology: The biologic basis for disease in adults and children (8th ed.). Elsevier.
Minutello, K., & Gupta, V. (2023). Cromolyn sodium. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK557473
National Heart, Lung, and Blood Institute. (2012). Asthma care quick reference: Diagnosing and managing asthma [Image]. NIH Publication No. 12-5075. https://www.nhlbi.nih.gov/sites/default/files/media/docs/asthma_qrg_0_0.pdf
National Heart, Lung, and Blood Institute. (2013a). Peak expiratory flow meter [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Peak_expiratory_flow_meter-pediatric_use.PNG
National Heart, Lung, and Blood Institute. (2013b). Spirometry testing [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Spirometry_NIH.jpg
National Heart, Lung, and Blood Institute. (2020). 2020 focused updates to the asthma management guidelines. https://www.nhlbi.nih.gov/resources/2020-focused-updates-asthma-management-guidelines
National Heart, Lung, and Blood Institute. (2021). Asthma action plan. (NIH Publication No. 20-HL-5251). https://www.nhlbi.nih.gov/sites/default/files/publications/Asthma-Action-Plan-2020_rev_508.pdf
National Heart, Lung, and Blood Institute. (2022.). Asthma. https://www.nhlbi.nih.gov/health-topics/asthma
O'Byrne, P. M., FitzGerald, J. M., Bateman, E. D., Barnes, P., Zhong, N., Keen, C., Jorup, C., Lamarca, R., Ivanov, S., & Reddel, H. K. (2018) Inhaled combined budesonide-formoterol as needed in mild asthma. New England Journal of Medicine, 378, 1865-76. https://doi.org/10.1056/NEJMoa1715274
Ortega, V. E., & Izquierdo, M. (2022a). Asthma. Merck Manual Professional Version. https://www.merckmanuals.com/professional/pulmonary-disorders/asthma-and-related-disorders/asthma
Ortega, V. E., & Izquierdo, M. (2022b). Drug treatment of asthma. Merck Manual Professional Version. https://www.merckmanuals.com/professional/pulmonary-disorders/asthma-and-related-disorders/drug-treatment-of-asthma#v31727208
Popovic-Grle, S., Stajduhar, A., Lampalo, M., & Rnjak, D. (2021). Biomarkers in different asthma phenotypes. Genes, 12(6), 801. https://doi.org/10.3390/genes12060801
Sayeedi, I., & Widrich, J. (2022). Methacholine challenge test. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK547716
Sharma, S., Hashmi, M. F., & Chakraborty, R. K. (2023). Asthma medications. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK531455
Shebl, E., & Chakraborty, R. K. (2022). Asthma in pregnancy. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK532283
Sinyor, B., & Perez, L. C. (2022). Pathophysiology of asthma. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK551579
Sobieraj, D. M., Baker, W. L., Nguyen, E., Weeda, E. R., Coleman, C. I., White, C. M., Lazarus, S. C., Blake, K. V., & Lang, J. E. (2018a). Association of inhaled corticosteroids and long-acting muscarinic antagonists with asthma control in patients with uncontrolled, persistent asthma: A systematic review and meta-analysis. JAMA, 319(14), 1473–1484. https://doi.org/10.1001/jama.2018.2757
Sobieraj, D. M., Baker, W. L., Weeda, E. R., Nguyen, E., Coleman, C. I., White, C. M., Lazarus, S. C., Blake, K. V., & Lang, J. E. (2018b). Intermittent inhaled corticosteroids and long-acting muscarinic antagonists for asthma. Agency for Healthcare Research and Quality. https://doi.org/10.23970/AHRQEPCCER194
Stickle, D. (2018). Spirometry: An integral tool for diagnosing and managing pediatric asthma. https://rtmagazine.com/public-health/pediatrics/spirometry-integral-tool-pediatric-asthma
Sverrild, A., Leadbetter, J., & Porsbjerg, C. (2021). The use of mannitol test as an outcome measure in asthma intervention studies: A review and practical recommendations. Respiratory Research, 22(287). https://doi.org/10.1186/s12931-021-01876-9
US Food and Drug Administration. (2012). MDI with attached spacer device [Image]. https://www.flickr.com/photos/fdaphotos/7349095984/in/photostream
Wang, Z., Pianosi, P., Keogh, K., Zaiem, F., Alsawas, M., Alahdab, F., Almasri, J. M., Mohammed, K., Larrea-Mantilla, L., Farah, W., Daraz, L., Barrionuevo, P., Gunjal, S., Prokop, L. J., & Murad, M. H. (2017). The clinical utility of fractional exhaled nitric oxide (FeNO) in asthma management. Agency for Healthcare Research and Quality. https://doi.org/10.23970/AHRQEPCCER197
Woods, A. D. (2023). Nursing2023 drug handbook (43rd ed.). Wolters Kluwer.
World Health Organization. (2023). Asthma. https://www.who.int/news-room/fact-sheets/detail/asthma