About this course:
This module discusses breast cancer, its risk factors, clinical features, common subtypes, and treatment modalities and reviews commonly used systemic treatments and side effects. It also includes a summary of early detection and screening guidelines to enhance nursing practice and improve clinical outcomes.
Course preview
Breast Cancer for RNs and LPNs
This module discusses breast cancer, its risk factors, clinical features, common subtypes, and treatment modalities and reviews commonly used systemic treatments and side effects. It also includes a summary of early detection and screening guidelines to enhance nursing practice and improve clinical outcomes.
By the completion of this learning activity, the nurse should be able to:
- discuss the epidemiology of breast cancer in the US and risk factors
- recognize the signs and symptoms of breast cancer, its primary classifications and subtypes, hormone receptivity, and other clinical features as components of breast cancer staging
- identify the various oral and intravenous treatment modalities for breast cancer, side effects, monitoring parameters, and warning precautions
- summarize breast cancer screening and early detection recommendations and guidelines for average-risk and high-risk women and discuss the role of nurses in cancer survivorship
Breast cancer is the second most common cancer diagnosed in women in the US and is one of the most common causes of cancer-related death. While breast cancer death rates declined 40% from 1989 to 2017, approximately 43,700 women and 530 men in the US are expected to die from breast cancer in 2023. The overall decline in mortality rate is primarily attributed to developments in earlier detection and screening practices, advancements in treatment modalities, and increased education and public awareness. Nurses should remain informed and current on effective strategies for preventing, screening, assessing, and treating breast cancer to help care for women at risk and ensure appropriate education. These practices must remain a priority to increase early detection and screening and reduce the associated morbidity and mortality (American Cancer Society [ACS], 2023a; 2023b; Breastcancer.org, 2023a).
Epidemiology
In the US, breast cancer accounts for approximately 30% of all new cancer cases diagnosed in females. There is a 1 in 8 (or 13%) chance a female will develop invasive breast cancer throughout her lifetime, and an estimated 297,790 new cases of invasive breast cancer will be diagnosed this year. While breast cancer is overwhelmingly a disease that affects women, it also occurs in men. The lifetime risk of breast cancer in males is about 1 in 833, and an estimated 2,800 new cases of invasive breast cancer will be diagnosed this year. While Black and White women are diagnosed with breast cancer at relatively comparable rates, Black women have the highest death rate. The risk of developing and dying from breast cancer is lower among Asian, Hispanic, and Native American women. Breast cancer is about 100 times less common among White males than White females. It is approximately 70 times less common among Black males than Black females. As with Black women, Black men with breast cancer have a poorer prognosis (ACS, 2023a, 2023b, 2023c, 2023d; Breastcancer.org, 2023a).
Breast cancer death rates have been steadily dropping, with an overall decline of 43% between 1989 and 2020. However, death rates among women in the US remain higher than those for any other cancer except lung cancer. There is an estimated 1 in 39 (2.5%) chance that a woman will die from breast cancer. The Surveillance, Epidemiology, and End Results (SEER) database evaluates five-year relative survival rates for breast cancer in the US based on how far the cancer has spread. The five-year relative survival rate for breast cancer is nearly 91%, which declines to 30% for those with metastatic disease (i.e., breast cancer that has spread to distant sites in the body). Aside from the extent and spread of the disease, survival rates vary based on the specific subtype and clinical features of breast cancer. There are more than 3.8 million female breast cancer survivors in the US (ACS, 2023a, 2023b, 2023c, 2023d; Breastcancer.org, 2023a).
Risk Factors
Although the risk for developing breast cancer is usually due to a combination of factors, the two most substantial risk factors include female gender and older age, as most breast cancers are diagnosed after age 50 (Centers for Disease Control and Prevention [CDC], 2022a). While most women with invasive breast cancer do not have identifiable risk factors, several elements and characteristics can influence their risk. Women with a strong family history of breast cancer, ovarian cancer, other hereditary cancer syndromes, or inherited changes in BRCA1 and BRCA2 genes are at high risk for breast cancer. The risk nearly doubles if a woman has a first-degree relative (such as a mother or sister) with breast cancer. However, about 85% of breast cancers occur in women with no family history (ACS, 2023a; Breastcancer.org, 2023a, 2023b). The risk for hormone receptor (HR)-positive breast cancers is increased in women with specific reproductive factors, such as those who have a long menstrual cycle history (early onset of menarche before age 12 or late onset of menopause after age 55), nulliparity, not breastfeeding, or having their first pregnancy after age 30. Postmenopausal hormone replacement therapy is a clinically significant risk factor, especially combined estrogen and progestin preparations. Other risk factors include a personal history of benign breast conditions, such as atypical hyperplasia, ductal carcinoma in situ (DCIS), lobular carcinoma in situ (LCIS), and prior chest irradiation (such as for the treatment of lymphoma). Unhealthy lifestyles characterized by excess alcohol consumption, obesity, and physical inactivity are associated with increased breast cancer risk, particularly among postmenopausal women with a high body mass index (BMI). In addition, dense breast tissue is an independent risk factor for breast cancer development, as it carries a relative risk of two to six times higher when compared to those with less-dense breasts (ACS, 2023a; Breastcancer.org, 2023a, 2023b; CDC, 2022a; Watkins, 2019).
BRCA1 and BRCA2 Gene Mutations
All women have BRCA1 and BRCA2 genes; these function as tumor suppressor genes to prevent cancer by regulating the growth and division of certain cell types. However, mutations in the BRCA genes disrupt these processes, increasing the propensity toward cancer development. Approximately 1 in 500 women in the US have a mutation in a BRCA gene. BRCA1 or BRCA2 mutations account for up to 10% of breast cancers, which tends to develop at a younger age. Women who inherit a BRCA1 mutation have up to a 72% lifetime risk of developing breast cancer, and those with a BRCA2 mutation have up to a 69% lifetime risk. Further, these mutations carry an increased risk of ovarian cancer. Nearly 30 in 100 women with a BRCA gene mutation will be diagnosed with ovarian cancer by the time they turn 70, compared with less than 1 in 100 women among the general population (CDC, 2023a; Breastcancer.org, 2023b).
Certain ethnic groups are at increased risk for inheriting BRCA mutations. According to the CDC (2023b), 1 in 40 Ashkenazi Jewish women inherit a BRCA gene mutation, which is why they notoriously have a higher risk of breast cancer. Among men, BRCA2 mutations are associated with a lifetime breast cancer risk of about 6.8%, whereas BRCA1 mutations are less frequently the culprit (Breastcancer.org, 2023a). Mutations in the BRCA1/BRCA2 genes are inherited in an autosomal dominant pattern (see Figure 1). One copy of the mutated gene in each cell is sufficient to increase the risk of developing breast cancer. Even though breast cancer is more common in women, the altered gene can be inherited from either the male or female parent. Each child of a parent with a BRCA1 or BRCA2 mutation has a 50% chance of inheriting the same gene mutation (US National Library of Medicine, 2021).
...purchase below to continue the course
Autosomal Dominant Inheritance Pattern
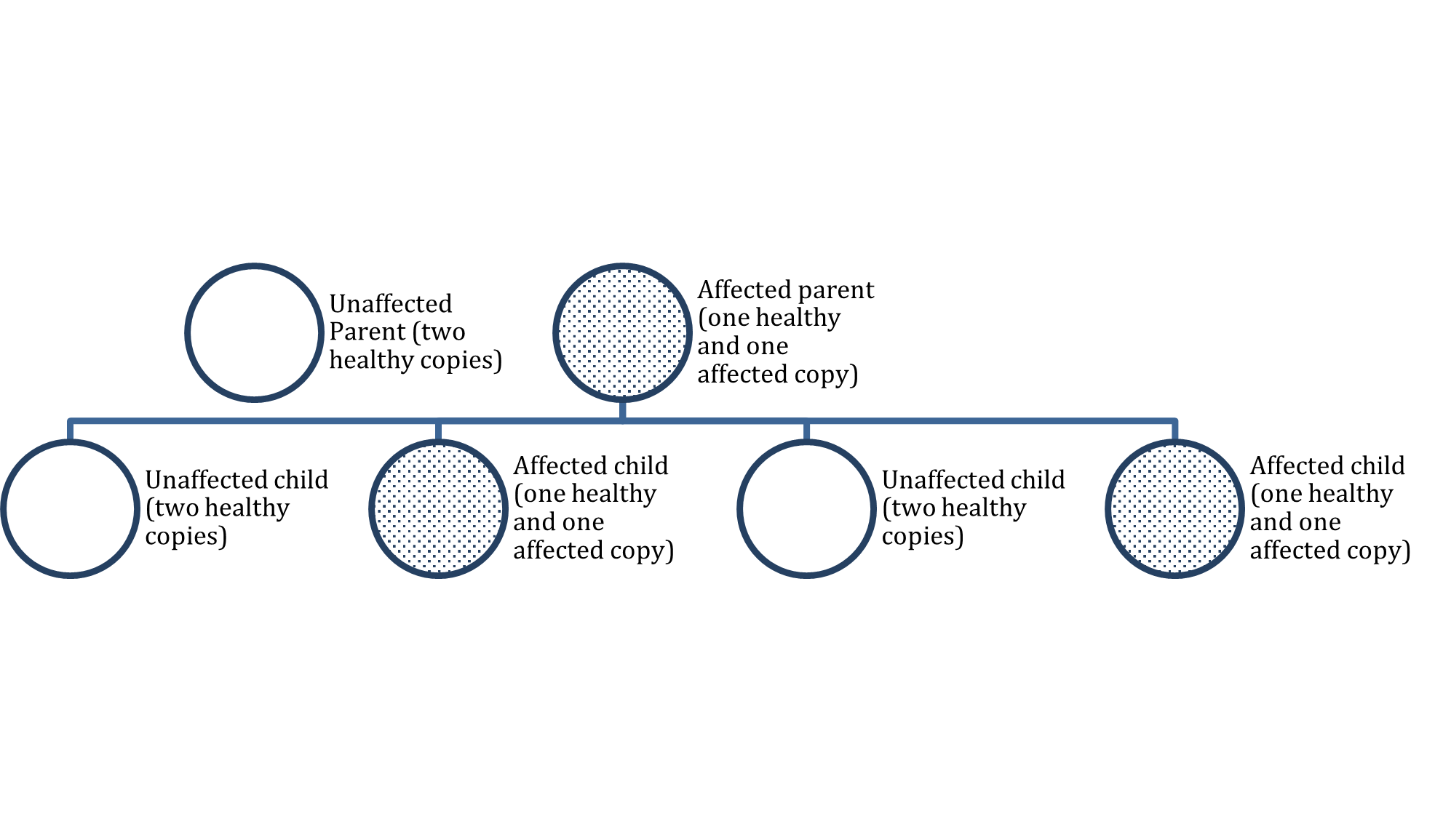
Pathophysiology
As demonstrated in Figure 2, breasts are composed of epithelial, stromal, and adipose (fat) tissues. Glandular tissue includes the ducts and lobules. Each breast contains about 15 to 20 lobes with many smaller subsections called lobules. The lobules lead to bulbs that generate milk, and these components are linked together by thin tubes called ducts. Most breast cancers arise from the cells forming the lobules and ducts as carcinoma in situ, an early-stage, noninvasive, abnormal proliferation of cells. LCIS is confined to the lobules, and DCIS is limited to the ducts. The lymphatic vessels of the breast flow in the opposite direction of blood flow, and they drain into lymph nodes. It is through the lymphatic vessels that breast cancer cells have the opportunity to metastasize (or spread) to lymph nodes. Most lymphatic vessels flow into the axillary lymph nodes, which are located in the armpit. Understanding the patterns of lymphatic drainage is essential, as when breast cancer metastasizes, it usually involves the first lymph node in the chain (McCance & Heuther, 2019).
Figure 2
Female Breast Anatomy
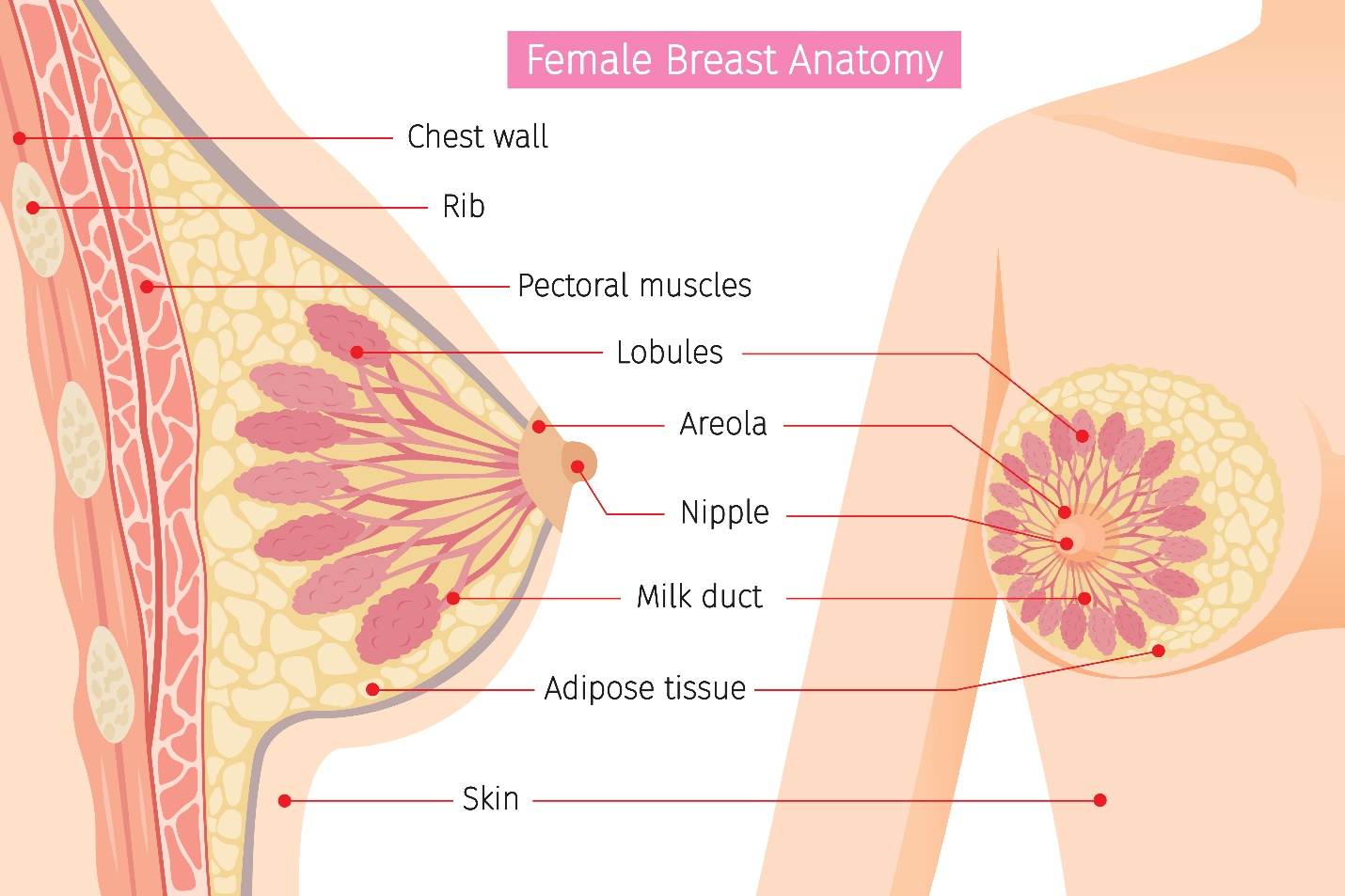
(iStock Illustration ID: 815081462)[MR2]
The pathophysiology of breast cancer is complex and multidimensional. Breast cancer is a heterogeneous disease with various molecular, genetic, and pathologic alterations. Breast cancer subtypes are classified by their unique clinical features, including histology, anatomical origin, tumor staging, hormone receptivity, and human epidermal growth factor receptor 2 (HER2) expression. The three most common types of receptors known to fuel most breast cancer growth include the estrogen receptor (ER), progesterone receptor (PR), and HER2 gene expression. Awareness of the presence or absence of these receptors is vital to determine the optimal evidence-based treatment regimen. Overexpression of HER2 has historically been associated with poorer overall survival rates, especially in patients with lymph node metastasis (Masoud & Pages, 2017). However, the advent of new HER2-targeted therapies has significantly shifted the treatment paradigm for these patients and improved survival (Patel et al., 2020). HR-positive, HER2 -negative (HR+/HER2-) is the most common breast cancer receptor subtype with an age-adjusted rate of 87.2 new cases per 100,000 women, based on 2016–2020 cases (SEER, 2023). More than two-thirds of patients with breast cancer are HR-positive and HER2-negative at diagnosis (Abdel-Razeq & Sharaf, 2022). Table 1 describes some of the most common breast cancer diagnoses based on histology.
Table 1
Common Breast Cancer Subtypes
Type | Clinical Features |
DCIS |
|
LCIS |
|
Invasive ductal carcinoma (IDC) |
|
Triple-negative breast cancer (TNBC) |
|
Inflammatory breast cancer (IBC) |
|
Metastatic breast cancer (MBC) |
|
(National Breast Cancer Foundation, n.d.b; Watkins, 2019; Yarbro et al., 2018)
Figure 3
Progression of Healthy Tissue to DCIS

(iStock Illustration ID: 1084191404)[MR3]
Signs and Symptoms
Early breast cancer generally has no signs or symptoms due to its small size; thus, it is most commonly identified on routine screening with mammography. If the breast tumor grows large enough, the most common signs include a unilateral, palpable mass with irregular borders. It may be a painless lump that is immobile or fixed to the skin and may or may not be accompanied by enlarged axillary lymph nodes. Some women may demonstrate changes to the breast caliber, texture, shape, asymmetry, open wounds, or lesions that arise spontaneously. Skin changes such as retraction, thickening, or dimpling of the skin on the breast may also occur. Nipple changes may also occur, including retraction of the nipple or spontaneous discharge. Blood or purulent discharge expelled from the nipple is an ominous sign of underlying pathology (Nettina, 2019).
Diagnostic Imaging
Most breast cancers in the US are diagnosed through mammography screenings. Standard (2-dimensional [2D]) mammography and ultrasonography (ultrasound) are the most common breast imaging tests. Mammography uses low-dose X-rays to identify tissue abnormalities suspicious of breast cancer. A standard mammogram is equivalent to approximately seven weeks of natural background radiation. In the US, mammography is performed as a screening modality (to be discussed later) or as a diagnostic test. Diagnostic mammography may be performed in follow-up to an abnormal screening mammogram or for patients with suspicious breast abnormalities, such as a palpable lump or skin changes. Diagnostic mammography differs from screening mammography in that it is supervised by a radiologist to tailor the additional views toward the area of suspicion to better characterize the abnormality. A breast biopsy is commonly advised for highly suspicious findings identified by diagnostic mammography. Diagnostic mammograms have lower sensitivity in women with increased breast tissue density and cannot differentiate between solid or cystic masses. A diagnostic mammogram is typically performed with a breast ultrasound in patients with dense breast tissue for superior visualization (ACS, 2022a; Koh & Kim, 2019; RadiologyInfo.org, 2023).
Three-dimensional (3D) mammography is known as tomosynthesis or digital breast tomosynthesis (DBT). It differs from standard mammography in that the machine moves in a small arc around the breast as it takes the low-dose X-rays. A computer fuses the images into thin slices, allowing a clearer depiction of the breast tissues. Some studies have found that 3D mammography can lower the chance of being called back for additional views and have a higher breast cancer detection rate in select patient groups. However, other studies have not reproduced the same statistically significant findings. Further, 3D mammograms are not available at all radiology centers and typically carry an added cost that may not be covered by insurance (ACS, 2022a; Lowry et al., 2020).
Ultrasound is a safe, noninvasive imaging modality that uses sound waves to generate images of internal body structures and carries no radiation exposure (Radiologyinfo.org, 2022). Magnetic resonance imaging (MRI) of the breast is particularly useful in women with dense or fibrotic breasts as it is superior in identifying abnormalities in soft tissues. It may also be used in specific circumstances, such as for patients with a prior history of breast cancer, to evaluate for contralateral disease, or surgical planning. MRIs are distinct from other forms of diagnostic imaging as they do not use X-rays or pose radiation exposure. MRIs utilize strong electromagnetic fields and radio waves to measure how much water is within different tissues within the body and generate detailed images of the underlying tissues (US Food & Drug Administration [FDA], 2018c).
Tissue Sampling
The only way to definitively confirm cancerous cells and establish the specific clinical features of the tumor is through tissue sampling, usually in the form of a breast biopsy. Tissue cells are examined under a microscope by a pathologist (National Breast Cancer Foundation, n.d.a). The main types of breast biopsies include core needle biopsy, surgical biopsy or excision, and fine-needle aspiration (FNA). While FNA is a lower-cost and minimally invasive procedure, it is substantially less accurate than the other two modalities and often requires a second diagnostic procedure. The preferred method is an image-guided core needle biopsy, associated with the highest diagnostic yield and minimal morbidity. It allows for proper histologic evaluation testing to be performed on the tissue. If a core needle biopsy is not feasible or is non-diagnostic, clinicians may consider proceeding with a surgical biopsy or excision (Yarbro et al., 2018).
Breast Cancer Staging
The American Joint Committee on Cancer (AJCC) directs breast cancer staging, which has evolved over the years. As demonstrated in Figure 4, the cancer stage depicts the extent of growth and spread within the body. It is a core element when developing the optimal evidence-based treatment regimen, as breast cancer prognosis and treatment decisions are predicated on cancer staging and its specific clinical features. The most updated AJCC cancer staging (8th edition) includes all of the clinical features listed in Table 2, which can be evaluated using the biopsy tissue (Breastcancer.org, 2022a).
Figure 4
Cancer Stages
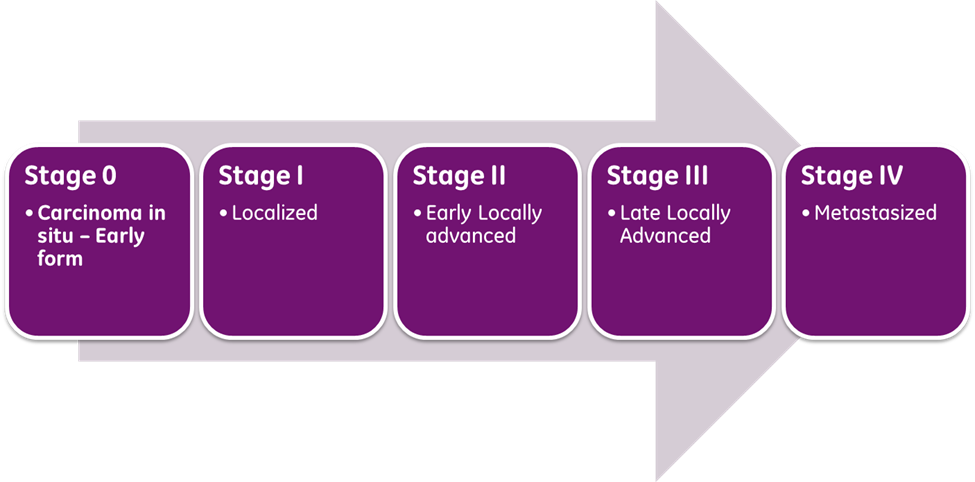
(Selchick, 2023)
Table 2
AJCC Breast Cancer Staging
TNM Staging System
|
|
Tumor Grade
|
|
Immuno- histochemistry (IHC) |
|
Oncotype DX Breast Recurrence Score
|
|
(Breastcancer.org, 2022a; 2023c; Koh & Kim, 2019; Exact Sciences, n.d.; Watkins, 2019)
Treatment Modalities
Treatment for breast cancer is typically multifactorial and involves the use of combined modalities. Most National Cancer Institute (NCI)-accredited institutions rely on the National Comprehensive Cancer Network (NCCN) evidence-based guidelines when formulating treatment plans. The NCCN (2023) is an alliance of leading cancer centers and world-renowned experts devoted to cancer care, research, and education. The NCCN provides evidence-based treatment guidelines for cancer type, pathology, genetics, staging, inheritance patterns, and several other specific features through rigorous clinical trial research, data compiled across institutions, and annual expert panel reviews. The guidelines are widely utilized in cancer care and direct medical decision-making throughout the disease trajectory (NCCN, 2023).
Surgery
Many patients will undergo surgery for breast cancer, but surgical options depend on the cancer’s clinical features, size, extent, and stage. The most common breast cancer surgical procedures include the following:
- Lumpectomy: the portion of the breast containing the cancerous cells is excised while leaving the remainder of the healthy breast tissue in place
- Axillary lymph node dissection (ALND): removal of affected lymph nodes in the axillary region; the amount removed depends on the extent of the cancer spread
- Mastectomy:
- Total (simple) mastectomy: the breast tissue, nipple, areola, and skin are removed, but not all the lymph nodes
- Modified radical mastectomy: the entire breast, most of the axillary region, and lymph nodes are removed, but the chest wall muscles under the breast remain
- Radical mastectomy: the most extensive type of mastectomy in which the entire breast is removed, ALND, and the chest wall muscles under the breast
- Partial mastectomy: the cancerous portion of the breast tissue is removed, along with some of the surrounding healthy tissue; similar to a lumpectomy, except more tissue is removed
- Nipple-sparing mastectomy: all breast tissue is removed, except the nipple is spared
- Prophylactic mastectomy: women with an increased risk for breast cancer due to BRCA1/2 genetic mutations or strong family history may consider bilateral prophylactic mastectomy as a risk reduction measure; some women who have had cancer in one breast may also opt to have the other breast removed as a means of prevention (Breastcancer.org, 2022a).
Chemotherapy
Surgery for breast cancer may be preceded by neoadjuvant chemotherapy and followed by adjuvant therapy. Neoadjuvant chemotherapy is administered before surgery to shrink the tumor and allow for less extensive surgery. In breast cancer patients, neoadjuvant chemotherapy may shrink the tumor enough so the breast surgeon can perform a lumpectomy instead of a mastectomy. Adjuvant therapy is given following surgery to eradicate any micro-metastases. Micro-metastases are a small collection of cancer cells detached from the original tumor and spread to other body parts. The danger with micro-metastases is that they can group and form additional cancerous tumors within the body. Adjuvant therapy is administered to prevent cancer recurrence (Yarbro et al., 2018). Chemotherapy, also called cytotoxic or antineoplastic therapy, encompasses a group of high-risk, hazardous drugs intending to destroy as many cancer cells with as minimal effect on healthy cells as possible. Chemotherapy interferes with the normal cell cycle, impairs DNA synthesis and cell replication, and prevents cancer cells from dividing, multiplying, or forming new cancer cells (Nettina, 2019).
A wide range of chemotherapy agents are used for breast cancer, typically administered in combinations of two or three drugs. Drug selection primarily depends on the stage of breast cancer and if the intent of treatment is curative or palliative. The most commonly used agents include the following:
- Taxanes, such as paclitaxel (Taxol), docetaxel (Taxotere), and albumin-bound paclitaxel (Abraxane)
- Anthracyclines or antitumor antibiotics, including doxorubicin (Adriamycin) and epirubicin (Ellence)
- Antimetabolites include 5-fluorouracil (5-FU), capecitabine (Xeloda), and gemcitabine (Gemzar)
- Alkylating agents include cyclophosphamide (Cytoxan) and carboplatin (Paraplatin)
Most chemotherapy agents are nonspecific, killing normal, healthy cells alongside cancer cells. As a result, they pose several side effects, which can also vary based on the specific agent (NCCN, 2023; Olsen et al., 2019).
Radiation Therapy
Radiation therapy is a localized treatment that uses radioactive energy to kill cancer cells. A precisely measured dose of ionizing radiation is delivered to the tumor with little injury to surrounding healthy tissue. Radiation induces cellular damage, causing biological changes in DNA, leading to cell death over days, weeks, and months. All healthy and cancer cells are vulnerable to the effects of radiation and may be injured or destroyed. Radiation renders cancer cells incapable of reproducing or spreading. Most non-cancerous cells can repair themselves and remain functional, whereas cancerous cells cannot (Yarbro et al., 2018). Radiation is generally performed following surgery to reduce the risk of cancer recurring in the breast, surrounding tissue, or lymph nodes. It may also be indicated for women following surgery if the tumor is large, if cancer cells were found in the lymph nodes, or if the surgical margins demonstrate signs of disease. Further, radiation therapy can also control symptoms and alleviate pain associated with metastatic disease, such as the bones or the brain (ACS, 2021).
The two primary types of radiation therapy for breast cancer include external beam radiation therapy (EBRT) and brachytherapy. EBRT is the most common; a machine aims for high-energy rays generated outside the body to a targeted area. EBRT usually begins about three to four weeks after surgery and is administered for a few minutes five days a week, lasting up to six weeks. Brachytherapy is a form of internal radiation delivered through an implantable device placed inside the breast. Brachytherapy delivers targeted radiation to the tumor bed (or the location where the cancer initially grew). Brachytherapy is often administered in only a few short treatments. Breast radiation is generally well-tolerated. The acute effects are usually transient, occur during treatment, are limited to the skin, and typically subside within two weeks of treatment cessation. Skincare is critical in patients undergoing radiation, as up to 95% of patients receiving radiation therapy will experience some skin reaction. Cumulative doses of radiation weaken the skin integrity, depleting stem cells from the basal layer of the epidermis, leading to varying degrees of radiodermatitis. Skin reactions generally begin around 7-14 days after the initiation of treatment, and the first signs include dryness and erythema. These symptoms may progress to bright red erythema, rash, and desquamation (sloughing off of the top layer of the skin). Many patients describe that the skin feels similar to a sunburn. Additional effects of breast radiation include fatigue and an increased risk for lymphedema (Nettina, 2019; Yarbro et al., 2018).
Hormonal Therapy
Hormonal treatments are targeted agents that block the hormones from reaching the cancer cells or prevent the body from producing the hormones (Nettina, 2019). Estrogen and progesterone receptors are key drivers in many breast cancers, making them amenable to hormone-blocking treatments to shrink or slow their growth. In addition to being a viable treatment, hormonal therapy can also be administered as chemoprevention to lower the risk of HR-positive breast cancer in individuals who are at high-risk (FDA, 2018b). Currently, there are four major types of hormonal therapy, which include selective estrogen receptor modulators (SERM), selective estrogen receptor down-regulators (SERD), luteinizing hormone-releasing hormones (LHRH), and aromatase inhibitors (AI), which are outlined in Table 3. The most common adverse effects of hormone treatment depend on the type of drug and can differ between males and females, but generally include hot flashes, night sweats, loss of libido, weight gain, joint aches or pains, mood changes, thinning or weakening of the bones (osteopenia or osteoporosis), and atrophic vaginitis (atrophy, loss of elasticity, dryness, and resulting irritation of the vaginal mucosa). Men who take hormonal therapy for breast cancer may experience hot flashes, erectile dysfunction, shrinking of the testicles, and gynecomastia (enlargement of breast tissue). Due to the impact of hormonal therapy on bone thinning, patients should be counseled on the importance of a calcium-rich diet with at least 1,200 mg of dietary calcium daily. Patients unable to consume the recommended amount of calcium in their diet should consider calcium supplementation. Nurses should also counsel patients on the importance of weight-bearing exercises to preserve bone strength and density. Exercise can also help reduce the severity of joint aches and pains associated with these medications (Olsen et al., 2019).
Table 3
Hormonal Treatments for Breast Cancer
Drug | Indications & Mechanism | Warnings & Precautions |
SERM
|
|
|
SERD
|
|
|
Oral SERD
|
|
|
LHRH
|
|
|
AI
|
|
|
(FDA, 2022b; ANI Pharmaceuticals, Inc., 2020; Breastcancer.org, 2022b; FDA, 2017, 2018b; Stemline Therapeutics, 2023)
Tamoxifen (Soltamax) is the oldest hormonal treatment for breast cancer, initially approved by the FDA in 1977. It is an estrogen agonist/antagonist approved for the treatment of HR-positive breast cancers as well as a risk-reducing agent in patients with DCIS. The recommended duration of therapy is 5 to 10 years (FDA, 2018b).
Targeted Therapies
Targeted therapies denote a few classes of novel treatment modalities that attack specific parts of cancer cells to prevent tumor development or shrink existing tumors. Growth factor receptors are proteins located on the cellular membranes. They connect the external and internal cellular environments and are essential for cellular growth and development. These drugs can block or turn off chemical signals that tell the cancer cell to grow and divide, starve the tumor by cutting off blood supply and/or by preventing the formation of new blood vessels, or carry toxins or poison directly to the cancer cells, killing them without harming healthy cells (Sengupta, 2017). In recent decades, developing specialized drugs to block growth factor receptors has been a central focus of breast cancer research. These drugs have revolutionized treatment options for patients living with advanced and metastatic breast cancer by fighting against drug resistance and increasing survival. The next section provides an overview of the major classes and types of targeted oral agents used to treat breast cancer (Breastcancer.org, 2022c; Masoud & Pages, 2017; NCCN, 2023).
Cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors
While hormone-blocking therapies are effective initially, nearly 50% of HR-positive breast cancers will develop resistance to it, leading to disease recurrence and limited clinical benefit. Several randomized clinical trials have demonstrated improvement in progression-free and overall survival since the arrival of CDK4/6 inhibitors. CDK4/6 pathways are key drivers in HR-positive, HER2-negative metastatic breast cancer. CDK4/6 inhibitors work by interrupting intracellular pathways and hormone signals that stimulate the growth and proliferation of cancer cells. The FDA approved CKD4/6 inhibitors in combination with endocrine therapy (i.e., an AI or fulvestrant (Faslodex)] for women with HR-positive, HER2 -negative advanced, or metastatic breast cancer. It is not fully understood how these three drugs compare with each other due to the lack of randomized, head-to-head comparison trials. While they are considered relatively equivalent in efficacy due to their overlapping activity patterns, emerging clinical trials are beginning to study their differences. The three available CDK4/6 inhibitors include palbociclib (Ibrance), abemaciclib (Verzenio), and ribociclib (Kisquali; (Abdel-Razeq & Sharaf, 2022; Johnston et al., 2022; Shah et al., 2018).
Oral CDK4/6 inhibitors are generally well tolerated. As a class, the most common adverse effects include bone marrow suppression (neutropenia, anemia, and thrombocytopenia), diarrhea, and fatigue. CDK4/6 inhibitors are associated with a low risk (~1.1%) of interstitial lung disease (ILD) and pneumonitis. Neutropenia is the most common adverse effect of ribociclib (Kisqali) and palbociclib (Ibrance), requiring close monitoring of blood counts. If significant neutropenia develops, dose interruption and/or reduction may be necessary. Ribociclib (Kisqali) has the highest affinity for CDK4 compared to the other CDK4/6 inhibitors. Therefore, it carries additional risks, including hepatobiliary toxicity and QTc interval prolongation; it requires monitoring hepatic function tests and serial ECGs at defined intervals and as clinically indicated. Diarrhea is the most common side effect of abemaciclib (Verzenio), affecting 85% to 90% of patients (Abdel-Razeq & Sharaf, 2022).
More recently, the role of CDK4/6 inhibitors has been expanded from the metastatic setting to the adjuvant setting for some patients with high-risk early breast cancer. In March 2023, the FDA approved abemaciclib (Verzenio) with endocrine therapy for the adjuvant treatment of adult patients with HR-positive, HER2-negative, node-positive, early breast cancer at high risk of recurrence. The label expansion was based on findings from the randomized, phase 3 monarchE trial that demonstrated adjuvant abemaciclib (Verzenio) reduces the risk for breast cancer recurrence within this high-risk population (FDA, 2023c; Johnston et al., 2022).
Phosphatidylinositol-3-kinase (PI3K) Inhibitor
Mutations in the PIK3CA gene in HR-positive and HER2 -negative breast cancers commonly cause cancer growth and resistance to hormonal treatments over time. Worldwide, nearly 40% of HR-positive, HER2 -negative breast cancer patients have a PIK3CA mutation. Alpelisib (Piqray) works by inhibiting PI3K, which increases estrogen receptor transcription in breast cancer cells. Combined with fulvestrant (Faslodex), it has demonstrated increased antitumor activity among patients with PIK3CA mutation. Alpelisib (Piqray) is FDA-approved only in conjunction with fulvestrant (Faslodex) in postmenopausal women (and men) with HR-positive, HER2 -negative, PIK3CA-mutated advanced or metastatic breast cancer (FDA, 2022c). In the SOLAR-1 trial, a double-blind, placebo-controlled multicenter phase 3 study in men and postmenopausal women with PIK3CA mutations, alpelisib (Piqray) nearly doubled progression-free survival (11.0 months versus 5.7 months in the placebo group; Andre et al., 2019). The most common side effects include diarrhea, nausea, fatigue, anorexia, and skin rash. The drug is also associated with severe adverse reactions, including hypersensitivity requiring drug discontinuation, severe skin reactions, hyperglycemia, and diarrhea. Patients require close monitoring of fasting blood glucose (FBG) and hemoglobin A1C (HbA1C), as there are strict guidelines for managing hyperglycemia while on this medication. Patients may require dose interruption, dose reduction, and the initiation of oral antihyperglycemics such as metformin (Glucophage) to optimize glucose control. Further, the cutaneous reactions can be severe, requiring close monitoring, dose modifications, and intervention with topical steroids. In severe cases, the drug may also need to be discontinued permanently (Andre et al., 2019; FDA, 2022c).
Mammalian Target of Rapamycin (mTOR) Pathway
The mTOR pathway is part of a complex intracellular pathway that serves as a key regulator of cell physiology in various cancers. It is a drug target in several types of cancers due to its links to different cellular and physiological functions involved in cellular growth, proliferation, and survival. Everolimus (Afinitor) is an mTOR inhibitor approved for use in postmenopausal women with HR-positive, HER2 -negative, advanced breast cancer in combination with exemestane (Aromasin) following a prior failure of treatment with letrozole (Femara) or anastrozole (Arimidex). The most common side effect of everolimus (Afinitor) is oral mucositis, which is inflammation, irritation, and swelling of the oral mucosa and lips, which can lead to painful ulcers that can develop within two weeks of starting the medication. Patients should be educated on routine oral hygiene practices to maintain the integrity of the oral mucosa, such as using soft-bristled toothbrushes and avoiding alcohol-based mouthwashes and hot foods or those that can cause oral irritation and dryness. Additional reported side effects include fatigue, anorexia, skin rash, diarrhea, and increased serum cholesterol levels. It carries a risk for pneumonitis, impaired wound healing, renal failure, and embryo-fetal toxicity. Everolimus (Afinitor) is also associated with a potentially increased risk for angioedema in patients taking concomitant angiotensin-converting enzyme (ACE) inhibitors (FDA, 2018a).
Poly ADP-ribose Polymerase (PARP) Inhibitors
The PARP enzyme is critical in cell growth, regulation, and repair of healthy and cancer cells. In cancer cells, it fixes DNA damage, essentially helping cancer cells repair themselves and survive (Olsen et al., 2019). PARP inhibitors interfere with the PARP enzyme, making it more difficult for cancer cells with a BRCA1 or BRCA2 mutation to repair DNA damage, thereby inducing cell death. PARP inhibitors have transformed the treatment of BRCA-positive cancers, such as ovarian and breast cancers (Ring & Modesitt, 2018). More recently, PARPI inhibitors are emerging as therapeutic options in metastatic BRCA-mutated prostate and pancreatic cancers. While a few drugs are in this class, olaparib (Lynparza) is the only FDA-approved PARP inhibitor for breast cancer. It was initially approved for patients with metastatic, HER2-negative breast cancer with an inherited BRCA1 or BRCA2 mutation and previously treated with chemotherapy. In 2022, the FDA expanded the label to include use in the adjuvant breast cancer setting for BRCA mutated, HER2-negative, high-risk early breast cancer (FDA, 2023b).
The most common side effects include anemia, neutropenia, fatigue, nausea, diarrhea or constipation, anorexia, and arthralgias. Olaparib (Lynparza) is also associated with a rare risk (less than 1.5%) of myelodysplastic syndrome (MDS), a bone marrow failure disorder, or acute myeloid leukemia (AML), a type of blood cancer. It is also associated with a slight risk (less than 1%) of pneumonitis and embryo-fetal toxicity. Patients should be counseled to alert their clinician before starting any new medications. In addition, patients should avoid grapefruit and Seville oranges, which can increase the effects of PARP inhibitors and lead to toxicity. Clinical research continues to explore PARP inhibitors’ effectiveness in additional settings (FDA, 2023b; NCCN, 2023).
Monoclonal Antibodies
Monoclonal antibodies are synthetic proteins that function like human antibodies in the immune system. They are primed to locate and attach to specific receptors on the surface of cancer cells, such as the HER2 receptor. HER2 -positive breast cancer is amenable to HER2-directed monoclonal antibody treatments, such as trastuzumab (Herceptin) or pertuzumab (Perjeta). These agents are recombinant DNA-derived humanized monoclonal antibodies that selectively bind with high affinity in a cell-based assay to the extracellular domain of the HER2 receptor, inducing cell death. They are approved for early-stage and advanced metastatic breast cancer whose tumors overexpress HER2 (ACS, 2022c; Olsen et al., 2019). Trastuzumab (Herceptin) was the first drug approved by the FDA in 1998 to treat HER2 -positive breast cancer. Trastuzumab (Herceptin) and pertuzumab (Perjeta) are intravenous infusions that may be combined with certain chemotherapy agents. In women treated with curative intent, these medications are given for 12 months; however, they may also be used in advanced and metastatic settings indefinitely as tolerated. Both drugs carry boxed warnings for cardiotoxicity, as they can lead to cardiac failure manifesting as decreased left ventricular dysfunction (LVEF) or congestive heart failure (CHF). Clinicians are advised to evaluate pre-treatment cardiac studies (i.e., echocardiogram or multiple-gated acquisition [MUGA] scan) to establish a baseline, followed by serial LVEF evaluations at defined intervals during treatment, and then every six months up to two years following the completion of treatment. There are specific guidelines for discontinuing or delaying therapy in case of a confirmed clinically significant decline in LVEF. In addition, these agents carry another boxed warning for embryo-fetal toxicity, as exposure can result in embryo-fetal death and birth defects. Diarrhea is a common side effect of pertuzumab (Perjeta), with the incidence ranging from 28% to 72%. Clinical studies demonstrate the incidence is highest during the first treatment, decreasing with subsequent cycles. Despite the high incidence of diarrhea in patients on pertuzumab (Perjeta), research demonstrates that it can usually be managed effectively without causing severe toxicity, treatment delays, or drug discontinuation (ACS, 2022c; Swain et al., 2017).
Researchers continue to study the HER2 gene extensively. Trastuzumab emtansine (Kadcyla) or T-DMI is a relatively novel agent antibody-drug conjugate of trastuzumab (Herceptin) that is linked to a cytotoxic agent. A conjugate is a compound that is combined with a chemotherapeutic agent as a means to induce a greater cell kill. Based on the KATHERINE trial, the risk of recurrence of invasive breast cancer or death was 50% lower with T-DM1 than with trastuzumab (Herceptin) alone. Due to these promising results, trastuzumab emtansine (Kadcyla) was approved by the FDA in 2019 for the treatment of early-stage, HER2 -positive breast cancer with residual disease after surgery, for initial adjuvant therapy and metastatic breast cancer (Minckwitz et al., 2019). Trastuzumab emtansine (Kadcyla) is administered intravenously every three weeks for 14 doses. It carries a boxed warning for hepatotoxicity, liver failure, and death. Clinicians must monitor the patient’s hepatic function before each dose, as some patients require dosing modifications or permanent discontinuation. It also carries the same boxed warnings as trastuzumab (Herceptin), including cardiotoxicity requiring serial LVEF monitoring and risk for embryo-fetal toxicity. While trastuzumab emtansine (Kadcyla) is generally well tolerated, the most common adverse reactions include fatigue, nausea, musculoskeletal pain, hemorrhage, thrombocytopenia, headache, increased transaminases, constipation, epistaxis, and arthralgias (FDA, 2022a).
In August 2022, the FDA approved fam-trastuzumab deruxtecan-nxki (Enhertu) for patients with unresectable or metastatic HER2-low breast cancer who received prior chemotherapy or have breast cancer that has come back within six months of completing treatment for early-stage breast cancer. HER2-low refers to tumors with lower levels of the HER2 protein on their surface. HER2-low expression accounts for about 50%–60% of all breast cancer diagnoses. Fam-trastuzumab deruxtecan-nxki (Enhertu) is a HER2-directed antibody and topoisomerase inhibitor conjugate (i.e., an antibody attached to chemotherapy). The antibody portion of the drug binds to HER2 on the cancer cell, allowing the chemotherapy to enter the cell. The FDA approval was based on the DESTINY-Breast04 randomized clinical trial, which enrolled 557 unresectable or metastatic HER2-low (defined as IHC 1+ or 2+/FISH negative) breast cancer patients. All patients had previously been treated for metastatic breast cancer with at least one other drug. Findings revealed that patients lived nearly twice as long without their cancer growing and lived six months longer than those treated with standard chemotherapy (Modi et al., 2022). This groundbreaking discovery is distinct from all other HER2-positive treatments because it is the first drug approved for HER2-low expression. Previously, only breast cancers with high levels of HER2 (e.g., HER2-positive breast cancer) had been shown to benefit from drugs targeting the HER2 receptor. While the findings of the DESTINY-Breast04 trial are remarkable, fam-trastuzumab deruxtecan-nxki (Enhertu) is not without risks and side effects. It can cause serious lung inflammation, including ILD and pneumonitis. Other possible side effects include neutropenia and left ventricular dysfunction (Daiichi Sankyo Co., 2022; NCI, 2022).
PD-1 Inhibitor. PD-1 is a transmembrane protein expressed on the surface of circulating T-cells, B-cells, and NK-cells and is used to recognize “self” antigens from “non-self.” PD-1 helps keep the immune response working properly. When PD-1 attaches to PD-L1, an inhibitory ligand expressed on some normal and cancer cells, it acts as an “off switch” to keep them from attacking other cells in the body. The binding of PD-1 to PD-L1 signals T-cells to leave the neighboring cells alone, including cancer cells. Some cancer cells have large amounts of PD-L1, which helps them evade immune attacks. PD-1 inhibitors are monoclonal antibodies developed to prevent the formation of this complex. They dislocate the immune system's brakes, and T cells are freed to attack cancer cells (ACS, 2022b; FDA, 2023a).
PD-L1 expression can be evaluated differently depending on the type of cancer. PD-L1 protein expression is determined by a scoring method called combined positive score (CPS) for breast cancer. CPS estimates the number of PD-L1–staining cells (i.e., tumor cells, lymphocytes, macrophages) relative to all viable tumor cells. The number of PD-L1 staining cells is divided by the total number of viable tumor cells, multiplied by 100. A minimum of 100 viable tumor cells in the PD-L1–stained slide are required for the specimen to be considered adequate evaluation, and the score is defined as CPS 0 (negative) to 100 (strongly positive; Merck & Co., Inc., 2023).
The only drug in this class currently FDA-approved for use in breast cancer is pembrolizumab (Keytruda). Pembrolizumab (Keytruda) is used to treat high-risk early-stage TNBC in combination with chemotherapy as neoadjuvant therapy and then continued as monotherapy after surgery. Additionally, pembrolizumab (Keytruda) is also FDA-approved for use in combination with chemotherapy for patients with locally recurrent unresectable or metastatic TNBC whose tumors express PD-L1 (CPS of 10 or greater; FDA, 2023a).
While pembrolizumab (Keytruda) is generally well tolerated, patients can experience severe and possibly life-threatening autoimmune-related adverse effects. Although an autoimmune reaction can affect any organ system, the most common reactions include the colon (colitis), liver (hepatitis), thyroid and adrenals (endocrinopathies), lung (pneumonitis), and skin (rash). The most common side effects include fatigue, nausea, anorexia, coughing, diarrhea, skin rash, and itching. Although rare, some patients may experience an infusion reaction during administration, and signs can include shortness of breath, wheezing, fever, chills, facial flushing, and skin rash (ACS, 2022b; FDA, 2023a).
The Early Detection of Breast Cancer
Breast Cancer Risk Assessment: Gail Model
Cancer risk assessment tools help estimate the risk for specific cancer types, guide evidence-based interventions to reduce that risk, and are intended for use as an adjunct to an individualized, comprehensive cancer risk assessment. The Gail Model is the most commonly used breast cancer risk assessment tool to estimate a woman’s five-year and lifetime risk for developing breast cancer (Smith et al., 2019). There are limitations to this tool, as it cannot calculate the risk in women under the age of 35, those with inherited mutations in BRCA1/2, or women with a history of invasive or in situ breast cancers. The tool is available online via the NCI (n.d.) at bcrisktool.cancer.gov.
Breast Cancer Screening
Screening tests are performed to diagnose cancer before it grows large enough to cause symptoms and is still treatable or potentially curable. Early detection refers to finding and diagnosing the disease earlier than if the person waited for symptoms to develop. Breast cancers identified during screening exams are more likely to be small and still confined to the breast. Tumor size at the time of diagnosis is directly related to mortality, as breast cancer survival is higher when detected before it has spread to other body parts. Breast cancer screening is most commonly performed through mammography. While screening recommendations vary based on organization, the ACS (2022a) recommends screening mammography beginning at age 40; this saves the most lives and the most years of life, and annual screening saves more lives than every other year (biennial). On May 9, 2023, the US Preventive Services Task Force (USPSTF, 2023) issued new draft recommendations for breast cancer screening that lowered the age women should start screening from 50 to 40 years old. During the same month, the American College of Radiology (ACR) issued new breast cancer screening guidelines, calling for earlier and more-intensive screening, particularly among high-risk women. While the ACR continues to support annual breast screening starting at age 40 for average-risk women, the new guidelines recommend that all women, particularly Black and Ashkenazi Jewish women, have a risk assessment by age 25 to determine if screening earlier than age 40 is needed (ACR, 2023; Monticciolo et al., 2023).
Annual mammography screening in premenopausal women is associated with a significantly decreased risk of identifying advanced breast cancer than screening performed every other year. Postmenopausal women do not receive the same benefits of annual screening unless they are currently receiving hormone replacement therapy for menopause; therefore, women 55 years or older can undergo screening every other year. The age to discontinue screening is not yet definitively established. Research demonstrates that continued screening may benefit certain women 75 years or older, depending on their mortality risk, comorbidities, overall health, and performance status (CDC, 2022b). All women should know the benefits, limitations, and potential harms of breast cancer screening. Women should check their breasts regularly to understand how they typically look and feel and report any changes to their healthcare provider immediately (ACS, 2022a; Smith et al., 2019).
ACS (2022a) Screening Recommendations for Average-Risk Women
- Women between 40 and 44 should have the option to start screening mammography annually.
- Women 45 to 54 should get mammograms every year.
- Women 55 and older can switch to a mammogram every other year or choose to continue yearly mammograms. Screening should continue as long as a woman is in good health and is expected to live at least ten more years.
- All women should understand what to expect when getting a mammogram for breast cancer screening – what the test can and cannot do.
ACS (2022a) Screening Recommendations for High-Risk Women
- Women at high risk for breast cancer should have a breast MRI and a mammogram annually, typically starting at age 30. This recommendation applies to women who meet the following criteria:
- lifetime risk of breast cancer of 20% to 25% or greater, according to risk assessment tools that are based mainly on family history
- known BRCA1 or BRCA2 gene mutation
- first-degree relative (i.e., parent, sibling, or child) with a BRCA1 or BRCA2 gene mutation but no genetic testing on themselves
- radiation therapy to the chest between the ages of 10 and 30
- Li-Fraumeni syndrome, Cowden syndrome, or Bannayan-Riley-Ruvalcaba syndrome, or have first-degree relatives with one of these syndromes
The ACR (2023) makes the following screening recommendations for high-risk women (Monticciolo et al., 2023):
- Women with genetics-based increased risk (including BRCA1 carriers), those with a lifetime risk of 20% or higher, and those exposed to chest radiation at a young age should have an MRI starting between ages 25 to 30 and annual mammography between ages 25 and 40, depending on the type of risk.
- Women with breast cancer before age 50 or a personal history of breast cancer and dense breasts should have annual breast MRIs.
Cancer Survivorship and the Fundamental Role of Nurses
According to the NCI Office of Cancer Survivorship (2022), “an individual is considered a cancer survivor from the time of diagnosis through the balance of their life. There are many types of survivors, including those living with cancer and those free of cancer.” As of January 2022, there are an estimated 18.1 million cancer survivors in the US, representing approximately 5.4% of the population. Of these, 3.8 million are breast cancer survivors, and these numbers are both projected to rise over the next decade due to continued developments in research and technology (ACS, 2023b; Miller et al., 2022; NCI Office of Cancer Survivorship, 2022). Cancer survivors have unique physical, emotional, and psychosocial needs that require the oversight of a skilled and knowledgeable healthcare team. Nurses serve pivotal roles in early cancer detection, managing survivors’ unique physical and psychosocial needs, and coordinating care throughout the cancer survivorship. Nurses are tasked with addressing and mitigating negative health behaviors associated with cancer and administering cancer-preventing vaccinations. They are the main drivers of screening and early detection practices for cancer. Nurses are uniquely positioned to address the needs of survivors to improve their quality of life, preserve health, and support well-being. Thus, nurses must develop the skillset to identify abnormal signs of cancer early, manage the late effects of cancer-related treatment, and recognize the signs of cancer recurrence to facilitate timely intervention. They support cancer survivors as they reintegrate into society following cancer treatment, often accompanied by significant changes in lifestyle and family dynamics, and pose new health concerns, including heightened screening and surveillance. Nurses serve fundamental roles in patient education and improving the quality and longevity of life throughout cancer survivorship (NCI Office of Cancer Survivorship, 2022; Nettina, 2019; Watson, 2021).
Learn more about cancer prevention, early detection, and survivorship with our NursingCE courses: Cancer Prevention and Early Detection and Cancer Survivorship.
References
Abdel-Razeq, H., & Sharaf, B. (2022). Expanding the clinical use of CDK4/6 inhibitors in the treatment of hormone receptor-positive, HER2-negative breast cancer from metastatic setting to adjuvant setting. Drug Des Devel Ther, 16, 727-735. http://doi.org/10.2147/DDDT.S356757
American Cancer Society. (2021). Radiation for breast cancer. https://www.cancer.org/cancer/breast-cancer/treatment/radiation-for-breast-cancer.html
American Cancer Society. (2022a). American Cancer Society recommendations for the early detection of breast cancer. https://www.cancer.org/cancer/breast-cancer/screening-tests-and-early-detection/american-cancer-society-recommendations-for-the-early-detection-of-breast-cancer.html
American Cancer Society. (2022b). Immune checkpoint inhibitors and their side effects. https://www.cancer.org/cancer/managing-cancer/treatment-types/immunotherapy/immune-checkpoint-inhibitors.html
American Cancer Society. (2022c). Monoclonal antibodies and their side effects. https://www.cancer.org/cancer/managing-cancer/treatment-types/immunotherapy/monoclonal-antibodies.html
American Cancer Society. (2023a). Cancer facts & figures, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
American Cancer Society. (2023b). Key statistics for breast cancer. https://www.cancer.org/cancer/types/breast-cancer/about/how-common-is-breast-cancer.html
American Cancer Society. (2023c). Key statistics for breast cancer in men. https://www.cancer.org/cancer/types/breast-cancer-in-men/about/key-statistics.html
American Cancer Society. (2023d). Survival rates for breast cancer. https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html
American College of Radiology. (2023). New ACR breast cancer screening guidelines call for earlier and more-intensive screening for high-risk women. https://www.acr.org/Media-Center/ACR-News-Releases/2023/New-ACR-Breast-Cancer-Screening-Guidelines-call-for-earlier-screening-for-high-risk-women
Andre, F., Ciruelos, E., Rubovszky, G., Compone, M., Loibl, S., Rugo, H. S., Iwata, H., Conte, P., Mayer, I. A., Kaufman, B., Yamashita, T., Lu, Y., Inoue, K., Takahashi, M., Papai, Z., Longin, A., Mills, D., Wilke, C., Hirawat, S., Juric, D., & SOLAR-1 Study Group. (2019). Alpelisib for PIK3CA-mutated, hormone-receptor-positive advanced breast cancer. New England Journal of Medicine, 380(20), 1929-1940. https://doi.org/10.1056/NEJMoa1813904
ANI Pharmaceuticals, Inc. (2020). Important safety information about Arimidex. https://www.arimidex.com/
Breastcancer.org. (2022a). Breast cancer stages. https://www.breastcancer.org/symptoms/diagnosis/staging
Breastcancer.org. (2022b). Lupron. https://www.breastcancer.org/treatment/druglist/lupron
Breastcancer.org. (2022c). Targeted therapies. https://www.breastcancer.org/treatment/targeted_therapies
Breastcancer.org. (2023a). Breast cancer facts and statistics. https://www.breastcancer.org/symptoms/understand_bc/statistics
Breastcancer.org. (2023b). Breast cancer risk factors. https://www.breastcancer.org/risk/risk-factors
Breastcancer.org. (2023c). Understanding your pathology report: HER2 status. https://www.breastcancer.org/pathology-report/her2-status
The Centers for Disease Control and Prevention. (2022a). What are the risk factors for breast cancer? https://www.cdc.gov/cancer/breast/basic_info/risk_factors.htm
The Centers for Disease Control and Prevention. (2022b). What is breast cancer screening? https://www.cdc.gov/cancer/breast/basic_info/screening.htm
The Centers for Disease Control and Prevention. (2023a). BRCA gene mutations. https://www.cdc.gov/cancer/breast/young_women/bringyourbrave/hereditary_breast_cancer/brca_gene_mutations.htm
The Centers for Disease Control and Prevention. (2023b). Jewish women and BRCA gene mutations. https://www.cdc.gov/cancer/breast/young_women/bringyourbrave/hereditary_breast_cancer/jewish_women_brca.htm
Daiichi Sankyo Co. (2022). Medication guide: ENHERTU® (fam-trastuzumab deruxtecan-nxki) for injection. https://daiichisankyo.us/prescribing-information-portlet/getPIContent?productName=Enhertu_Med&inline=true
Exact Sciences. (n.d.). Oncotype DX breast recurrence score® test. Retrieved June 30, 2023, from https://precisiononcology.exactsciences.com/healthcare-providers/treatment-determination/breast-cancer/oncotype-dx-breast-recurrence-score
Johnston, S. R., Toi, M., O’Shaughnessy, J., Rastogi, P., Campone, M., Neven, P., Huang, C., Huober, J., Jaliffe, G. G., Cicin, I., Tolaney, S. M., Goetz, M., Rugo, H. S., Senkus, E., Testa, L., & Del Mastro, L., Shimizu, C., Wei, R., Shahir, A., Munoz, M., San Antonio, B., Andre, V., Herbeck, N., Martin, M. (2022). Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): Results from a preplanned interim analysis of a randomized, open-label, phase 3 trial. The Lancet, 24(1), 77-90. https://doi.org/10.1016/S1470-2045(22)00694-5
Koh, J., & Kim, M. J. (2019). Introduction of a new staging system of breast cancer for radiologists: An emphasis on the prognostic stage. Korean Journal of Radiology, 20(1), 69-82. https://pubmed.ncbi.nlm.nih.gov/30627023/
Lowry, K. P., Coley, R. Y., Miglioretti, D. L., Kerlikowske, K., Henderson, L. M., Onega, T., Sprague, B. L., Lee, J. M., Herschorn, S., Tosteson, A. N. A., Rauscher, G., & Lee, C. I. (2020). Screening performance of digital breast tomosynthesis vs. digital mammography in community practice by patient age, screening round, and breast density. JAMA Network Open, 3(7), e2011792. https://doi.org/10.1001/jamanetworkopen.2020.11792
Masoud, V., & Pages, G. (2017). Targeted therapies in breast cancer: New challenges to fight against resistance. World Journal of Clinical Oncology, 8(2), 120-134. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5385433/
McCance, K. L., & Heuther, S. E. (2019). Pathophysiology: The biologic basis for disease in adults and children. (8th ed.). Elsevier.
Merck & Co., Inc. (2023). PD-L1 testing & scoring. https://www.keytrudahcp.com/biomarker-
testing/pd-l1/#defining-pd-l1-testing
Miller, K. D., Nogueira, L., Devasia, T., Mariotto, A. B., Yabroff, K. R., Jemal, A., & Kramer, J. (2022). Cancer treatment and survivorship statistics, 2022. CA: A Cancer Journal for Clinicians, 72(5), 409-436. https://doi.org/10.3322/caac.21731
Minckwitz, G., Huang, C., Mano, M., Loibl, S., Mamounas, E., Untch, M., Wolmark, N., Rastogi, P., Schneeweiss, A., Redondo, A., Fischer, H., Jacot, W., Conlin, A., Arce-Salinas, C., Wapnir, I., Jackisch, C., DiGiovanna, M., Fasching, P., Crown, J., … Geyer, C. (2019). Trastuzumab emtansine for residual invasive HER2-positive breast cancer. New England Journal of Medicine, 380(7),617-628. https://doi.org/10.1056/NEJMoa1814017
Modi, S., Jacot, W., Yamashita, T., Sohn, J., Vidal, M., Tokunaga, E., Tsurutani, J., Ueno, N. T., Prat, A., Chae, Y. S., Lee, K. S., Niikura, N., Park, Y. H., Xu, B., Wang, X., Gil-Gil, M., Li, W., Pierga, J., Im, S., Moore, H. C. F., Rugo, H. S., … Destiny-Breast04 Trial Investigators. (2022). Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. New England Journal of Medicine, 387(1), 9-20. https://doi.org/10.1056/NEJMoa2203690
Monticciolo, D. L., Newell, M. S., Moy, L., Lee, C. S., & Destounis, S. V. (2023). Breast cancer screening for women at higher-than-average risk: Updated recommendations from the ACR. Journal of the American College of Radiology, 20(6). https://doi.org/10.1016/j.jacr.2023.04.002
National Breast Cancer Foundation. (n.d.a). Diagnosis. Retrieved June 29, 2023, from https://www.nationalbreastcancer.org/breast-cancer-diagnosis/
National Breast Cancer Foundation. (n.d.b). Types of breast cancer. Retrieved June 29, 2023, from https://www.nationalbreastcancer.org/types-of-breast-cancer/
National Cancer Institute. (n.d.). The breast cancer risk assessment tool. Retrieved June 29, 2023, from https://bcrisktool.cancer.gov/index.html
National Cancer Institute Office of Cancer Survivorship. (2022). Statistics and graphs. https://cancercontrol.cancer.gov/ocs/statistics#definitions
National Comprehensive Cancer Network. (2023). NCCN clinical practice guidelines in oncology (NCCN Guidelines®) breast cancer version 4.2023 - March 23, 2023. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
Nettina, S. M. (2019). Lippincott manual of nursing practice (11th ed.). Wolters Kluwer.
Olsen, M., LeFebvre, K., & Brassil, K. (2019). Chemotherapy and immunotherapy guidelines and recommendations for practice. (1st Ed.). Oncology Nursing Society.
Patel, A., Unni, N., & Peng, Y. (2020). The changing paradigm for the treatment of HER2-positive
breast cancer. Cancers (Basel), 12(8), 2081. https://doi.org/10.3390/cancers12082081
RadiologyInfo.org. (2022). General ultrasound.
https://www.radiologyinfo.org/en/info.cfm?pg=genus
RadiologyInfo.org. (2023). Mammography.
https://www.radiologyinfo.org/en/info.cfm?pg=mammo
Ring, K. L., & Modesitt, S. C. (2018). Hereditary cancers in gynecology. Obstetrics and Gynecology Clinics, 45(1), 155-173. https://doi.org/10.1016/j.ogc.2017.10.011
Selchick, F. (2023). Cancer stages [image].
Sengupta, S. (2017). Cancer nanomedicine: Lessons for immune-oncology. Trends Cancer, 3(8), 551-560. https://doi.org/10.1016/j.trecan.2017.06.006.
Shah, M., Nunes, M. R., & Stearns, V. (2018). CKD4.6 inhibitors: Game changes in the management of hormone-receptor-positive advanced breast cancer? Oncology (Williston Park), 32(5), 216-222. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6424488/pdf/nihms-1010845.pdf
Smith, R. A., Andrews, K. S., Brooks, D., Fedewa, S. A., Manassaram-Baptiste, D., Saslow, D., & Wender, R. C. (2019). Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA: A Cancer Journal for Clinicians, 69(3), 184-210. https://doi.org/10.3322/caac.21557
Stemline Therapeutics, Inc. (2023). ORSERDU: Elacestrant: Important safety information. https://www.orserduhcp.com/
Surveillance, Epidemiology, and End Results. (2023). Cancer stat facts: Female breast cancer subtypes. https://seer.cancer.gov/statfacts/html/breast-subtypes.html
Swain, S. M., Schneeweiss, A., Gianni, L., Gao, J. J., Stein, A., Waldron-Lynch, M., Heeson, S., Beattie, M. S., Yoo, B., Cortes, J., & Baselga, J. (2017). Incidence and management of diarrhea in patients with HER2-positive breast cancer treated with pertuzumab. Annals of Oncology, 28(4), 761-768. https://doi.org/10.1093/annonc/mdw695
US Food & Drug Administration. (2017). Highlights of prescribing information: Faslodex® (fulvestrant). https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021344s034lbl.pdf
US Food & Drug Administration. (2018a). Highlights of prescribing information: Afinitor®(everolimus). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/022334s040,203985s013lbl.pdf
US Food & Drug Administration. (2018b). Highlights of prescribing information: Soltamox® (tamoxifen citrate). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021807s005lbl.pdf
US Food & Drug Administration. (2018c). MRI (magnetic resonance imaging). https://www.fda.gov/radiation-emitting-products/medical-imaging/mri-magnetic-resonance-imaging
US Food & Drug Administration. (2022a). Highlights of prescribing information: Kadcyla® (ado-trastuzumab emtansine). https://www.gene.com/download/pdf/kadcyla_prescribing.pdf
US Food & Drug Administration. (2022b). Highlights of prescribing information: LUPRON DEPOT (leuprolide acetate for depot suspension). https://www.rxabbvie.com/pdf/lupronuro_pi.pdf
US Food & Drug Administration. (2022c). Highlights of prescribing information: PIQRAY® (alpelisib). https://www.novartis.com/us-en/sites/novartis_us/files/piqray.pdf
US Food & Drug Administration. (2023a). Highlights of prescribing information: KEYTRUDA® (pembrolizumab). https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf
US Food & Drug Administration. (2023b). Highlights of prescribing information: LYNPARZA® (olaparib). https://den8dhaj6zs0e.cloudfront.net/50fd68b9-106b-4550-b5d0-12b045f8b184/00997c3f-5912-486f-a7db-930b4639cd51/00997c3f-5912-486f-a7db-930b4639cd51_viewable_rendition__v.pdf
US Food & Drug Administration. (2023c). Highlights of prescribing information: VERZENIO® (abemaciclib). https://uspl.lilly.com/verzenio/verzenio.html#pi
US National Library of Medicine. (2021). Breast cancer. https://ghr.nlm.nih.gov/condition/breast-cancer#sourcesforpage
US Preventive Services Task Force. (2023). Draft recommendation statement: Breast cancer: Screening (May 09, 2023). https://www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/breast-cancer-screening-adults
Watkins, E. J. (2019). Overview of breast cancer. American Academy of Physician Assistants, 32(10), 13-17. https://doi.org/10.1097/01.JAA.0000580524.95733.3
Watson, L., Maheu, C., Champ, S., & Fitch, M. I. (2021). Empowering oncology nurses through knowledge and practice to improve transitions following treatment and survivorship care. Asia Pacific Journal of Oncology Nursing, 8(5), 555-559. https://doi.org/10.4103/apjon.apjon-215
Yarbro, C. H., Wujcik, D., & Gobel, B. H. (Eds.). (2018). Cancer nursing: Principles and practice. (8th ed.). Jones & Bartlett Learning.