About this course:
This course aims to ensure that advanced practice registered nurses (APRNs) understand the pathophysiology, risk factors, and current diagnostic and management guidelines of the primary types of diabetes.
Course preview
Diabetes (for APRNs)
This course aims to ensure that advanced practice registered nurses (APRNs) understand the pathophysiology, risk factors, and current diagnostic and management guidelines of the primary types of diabetes.
After this course, learners will be prepared to:
- describe the statistical data regarding diabetes in the US, including prevalence and significance
- explain the pathophysiology of the primary forms of diabetes
- discuss the risk factors for diabetes
- explore the diagnostic guidelines for diabetes
- define evidence-based care for the management of diabetes
- recognize complications of diabetes and opportunities to decrease the risk
Each year in the US, about 1.4 million individuals are diagnosed with diabetes mellitus (DM). The incidence of diabetes peaked in 2008 and has been slowly decreasing since. In 2019, an estimated 37.3 million individuals of all ages, or 11.3% of the population, in the US had some form of diabetes, both diagnosed and undiagnosed (The Centers for Disease Control and Prevention [CDC], 2022j). In the US, $1 out of every $4 healthcare dollars is spent on diabetes, making it the most expensive chronic condition. The direct costs of diabetes care equate to $237 billion per year, with an additional cost of $90 billion in lost productivity. Of the total cost of diabetes, 61% is paid by Medicare for those over 65 (CDC, 2022f).
Statistical Data in the US
There is a tremendous amount of data gathered each year related to diabetes. Identifying at-risk individuals and populations is essential to provide targeted education and prevention information. Among individuals aged 18 years or older in the US, when categorized by ethnic group, Native Americans and Alaska Natives had the highest rate of diagnosed diabetes between 2018 and 2019, totaling 14.5%. The next highest groups were non-Hispanic Black individuals (prevalence rate of 12.1%), Hispanic individuals (11.8%), Asian Americans (9.5%), and non-Hispanic White individuals (7.4%). When US adults with diabetes were categorized by education level, those patients with less than a high school diploma had the highest prevalence rate at 13.4%. Those with a high school diploma had a prevalence rate of 9.2%, followed by those with more than a high school diploma at 7.1% (CDC, 2022i, 2022j).
While the national median indicates that 8.7% of the adult population in the US is diagnosed with diabetes, the state with the highest prevalence is West Virginia at 13.4%, and the lowest is Colorado at 6.6%. The territory of Guam has a prevalence of 15.4%, and Puerto Rico has a prevalence of 13.1%. Most southern states have double-digit prevalence rates, including Texas (12.0%), Louisiana (12.9%), Arkansas (12.4%), Mississippi (12.9%), Alabama (12.7%), Georgia (11.7%), South Carolina (11.6%), Kentucky (12.1%), and Tennessee (12.2%; 2022h). In 2019, approximately 283,000 individuals diagnosed with diabetes were children and adolescents under 20; of those, 244,000 were diagnosed with type 1 diabetes mellitus (T1DM; CDC, 2022k). T1DM and type 2 diabetes mellitus (T2DM) are rising in children, with an increase of 4.8% in T2DM versus a 1.9% increase in T1DM. Non-Hispanic White children were diagnosed with T1DM at a rate of 23.9 per 100,000, versus non-Hispanic Black children at 14.7 per 100,000, Hispanic children at 13.7 per 100,000, and Native American children at 6.6 per 100,000. Conversely, Native American children were diagnosed with T2DM at a rate of 22.6 per 100,000, Hispanic children at 13.3 per 100,000, non-Hispanic Black children at 20 per 100,000, and non-Hispanic White children at 4.4 per 100,000 (Divers et al., 2020).
In addition to the high number diagnosed with some form of diabetes, the CDC estimates that over 96 million US adults (38%) over age 18 have prediabetes; a significant number remain undiagnosed and unaware of their risk or condition. Only 19% of adults report being told by their provider that they have prediabetes. This leaves an opportunity for education, screening, and prevention measures (2022j). In 2010, the National Diabetes Prevention Program (DPP) was created to address the increased prevalence of prediabetes and T2DM. The National DPP offers interventions and support to individuals to facilitate lifestyle changes, including dietary changes and increased physical activity. Participation in structured lifestyle change programs has been shown to decrease the risk of developing T2DM in those with prediabetes by 58%, or 71% for those individuals over 60. As of 2022, over 640,000 adults had participated in the program (CDC, 2022k).
The most recent data on the economic burden of diabetes care is based on a report released by the ADA in 2018. Per the report, the cost of medication and supplies to treat diabetes was $29.3 billion. An additional $37.3 billion was spent on cardiovascular-related illnesses associated with diabetes. Patients over 65 contribute significantly to the growing economic impact of diabetes. Studies have shown that the average hospital stay is longer for patients with diabetes than for those without. There is also increased utilization of all healthcare services among patients with diabetes. There was a projected 40.3 million hospital days incurred by patients with diabetes in 2017, and about one-fourth of all nursing/residential facility days are incurred by patients with diabetes. Almost half of all physician office visits, emergency department visits, hospital outpatient visits, and medication prescriptions incurred by patients with diabetes are attributed to their diabetic diagnoses. In addition to the monetary cost of diabetes, diabetes also negatively impacts the quality of life. It is associated with disability leading to inability to work/lost productivity, premature death, pain, and suffering of the patient and their loved ones (ADA, 2018).
Pathophysiology and Risk Factors of Diabetes
DM is a chronic disease impacting multiple body systems due to abnormal insulin production, impaired insulin utilization, or both. If inadequately treated, DM can lead to severe complications; DM is the leading cause of end-stage renal disease, blindness, and non-traumatic lower-limb amputations. DM is a significant contributing factor to hypertension (HTN, elevated blood pressure), cardiovascular disease (CVD), and stroke, which all lead to premature death (Harding, 2020).
Diabetes is caused by a combination of genetic, autoimmune, and environmental factors, including viruses and obesity. Normal insulin metabolism occurs through the continuous release of insulin by the ß (beta)-cells in the islets of Langerhans of the pancreas (Figure 1). Insulin synthesis begins with its precursor, proinsulin. Enzymes split proinsulin to make insulin and C-peptide in equal amounts. This byproduct, C-peptide, is valuable when assessing pancreatic ß-cell function as it can be measured in the urine and blood. The average amount of insulin secreted daily by a healthy adult is 40-50 U or 0.6 U/kg of body weight. Insulin acts as an anabolic or storage hormone in the body. The insulin secreted with food intake promotes glucose transport into the cell for energy by unlocking receptor sites in the skeletal muscle and adipose tissue. Skeletal muscles and adipose tissue are considered insulin-dependent; the brain, liver, and blood cells do not depend on insulin and only require an adequate supply of glucose for normal functioning. While liver cells (hepatocytes) are not insulin-dependent, they have receptor sites that promote glucose uptake into the liver and subsequent glycogenesis (converting glucose to glycogen). As blood glucose (BG) increases after a meal or food intake, glucose is stored as glycogen in the liver and muscle tissue. Concurrently, insulin secretion inhibits gluconeogenesis (glucose production from non-sugar...
...purchase below to continue the course
Figure 1
The Pancreas
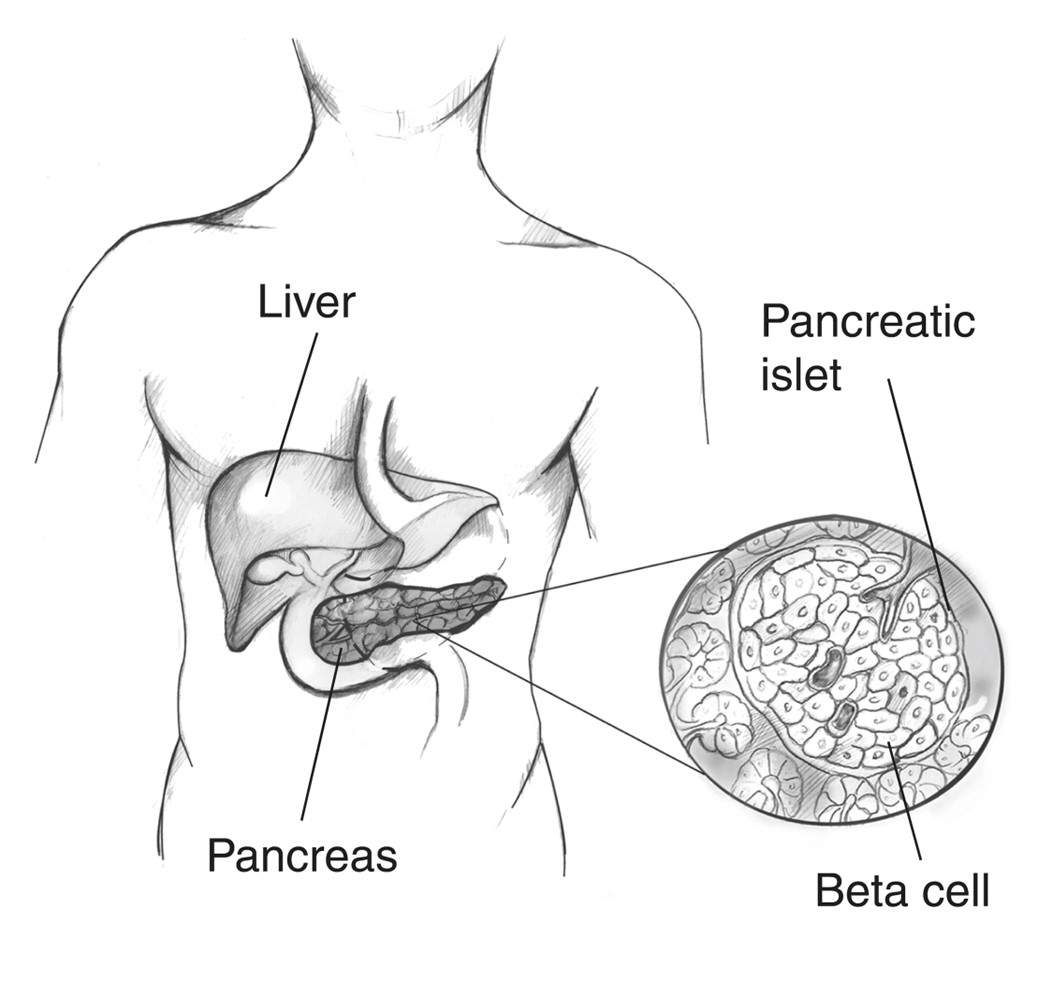
(National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK], n.d.-a)
Counter-regulatory hormones such as glucagon, epinephrine, growth hormone, and cortisol work to oppose the effects of insulin (Figure 2). They increase BG by stimulating the production of glucose and liver output and decrease the movement of glucose into the cells. Insulin secretion is designed to maintain a stable BG level of 70-120 mg/dL based on the time of day and the time since the last meal. The normal range of BG levels is typically maintained by regulating the release of glucose for energy during periods of fasting, food intake, and the production and release of insulin and counter-regulatory hormones (Harding, 2020).
Figure 2
The Regulation of Blood Glucose
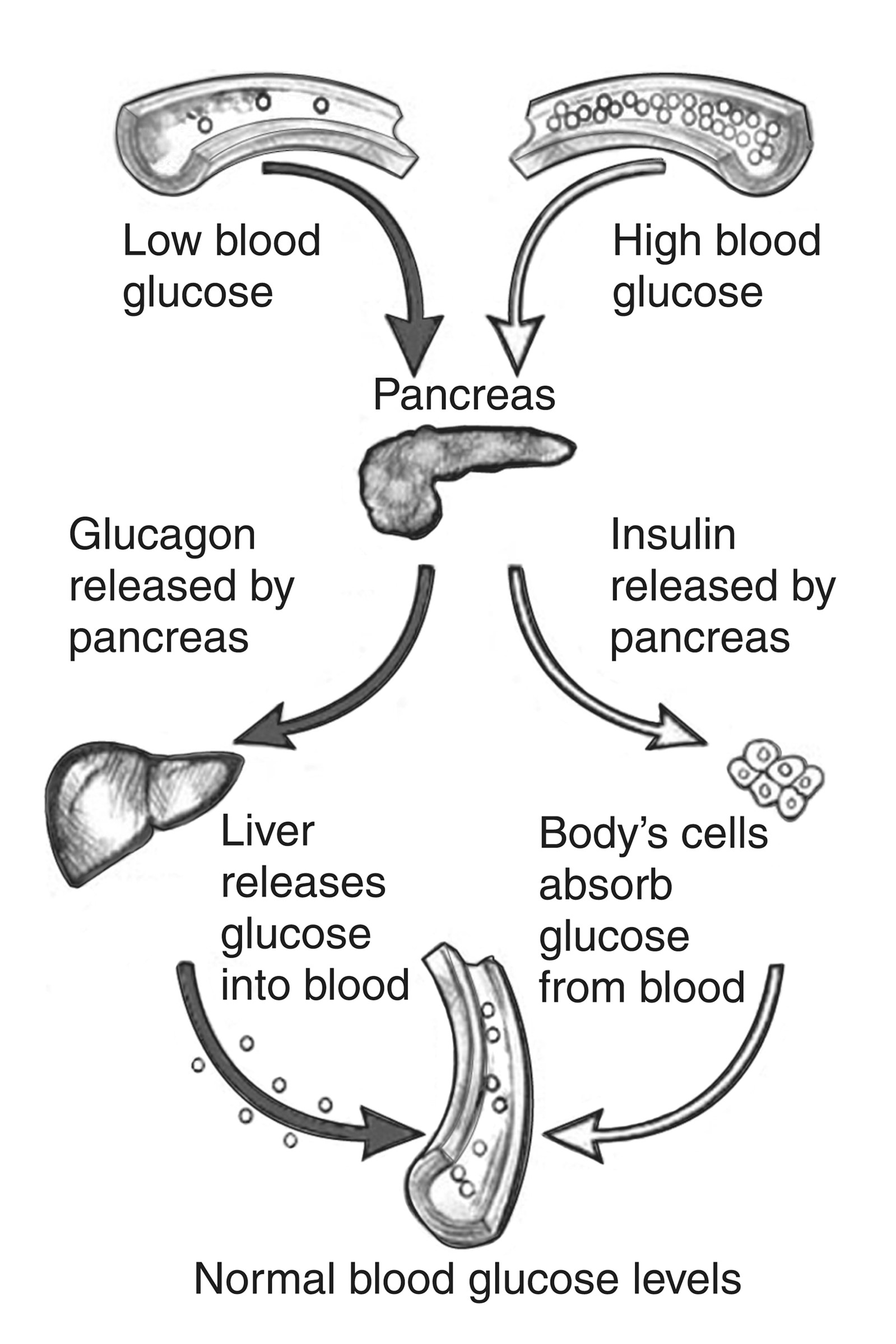
(NIDDK, n.d.-c)
Type 1 DM is characterized by the autoimmune destruction of the pancreatic ß-cells leading to the total absence of insulin production. A genetic predisposition is typically compounded by exposure to a virus that contributes to this autoimmune condition. Autoantibodies to the islet cells cause a decrease in normal function before other symptoms of T1DM appear. The genetic component of T1DM is primarily (40-50%) related to the human leukocyte antigens (HLAs), also called the major histocompatibility complex (MHC). Specific HLA genotypes, the combination of HLA alleles inherited from two genetic parents, increases the risk of developing T1DM. When these at-risk individuals are exposed to viral infections, the ß-cells of the pancreas can be destroyed. Twin studies indicate that T1DM is found in non-identical twins at the same rate as siblings; however, in cases of identical twins, both twins were diagnosed with T1DM in over 70% of cases (Harding, 2020; Redondo et al., 2018).
About 5-10% of all people with diabetes have T1DM. T1DM affects children and adults but is usually diagnosed in children, teens, and young adults; of all pediatric diabetes cases, 80% are due to T1DM, and 30% of patients are diagnosed during adulthood. Peak incidence is between the ages of 11 and 13 and rarely before age 4. Genetic factors contribute strongly to the development of T1DM, with less than a 0.4% risk in the general population (Khardori, 2022; Redondo et al., 2018). Fathers with T1DM have a 1 in 17 (or 5.8%) risk of passing it to their children. Mothers with T1DM have a lower risk of passing T1DM to their children: 1 in 25 if the mother is under 25, and 1 in 100 if the mother is over 25. Young adults considering having children should be educated on this risk (ADA, n.d.-d).
As previously discussed, there are other risk factors and triggers for the development of T1DM, including viral illnesses, autoimmune responses, and unknown or poorly understood factors. While diet and lifestyle do not cause T1DM, they are important components in disease management (CDC, 2022c). Risk factors for T1DM:
- genetic predisposition
- having a first-degree relative with T1DM; the risk to siblings ranges from 1 in 12-35, with the risk increasing if the sibling was diagnosed before 7 years old
- non-Hispanic White Americans are more likely to develop T1DM than non-Hispanic Blacks or Hispanic/Latin Americans (CDC, 2022c)
- viral illnesses
- enteroviruses
- mumps
- cytomegalovirus (CMV)
- rotavirus
- influenza
- congenital rubella
- human parvovirus
- coronavirus (Khardori, 2022; Rewers et al., 2018)
- autoimmune factors
- there is a relation between T1DM and other autoimmune diseases such as thyroid diseases, including Graves’ disease or Hashimoto's thyroiditis (15-30%), Addison's disease (0.5%), vitiligo (1-7%), rheumatoid arthritis (1.2%), type A gastritis (15%), systemic lupus erythematosus (1.15%), and celiac disease (3-12%)
- due to the association between T1DM and other autoimmune diseases, it is recommended that patients with T1DM undergo regular screening for autoimmune disorders (Frommer & Kahaly, 2020)
Another form of T1DM, idiopathic diabetes, is inherited, has no known etiology, and is not HLA-associated. There are only a small number of people with this type of diabetes; it is most common in individuals of Hispanic, African, or Asian descent. These patients have permanent insulinopenia and are at an increased risk of developing diabetic ketoacidosis (DKA). However, when tested for antibodies, they do not have any ß-cell autoantibodies or other autoimmune markers. A third form of T1DM is referred to as latent autoimmune diabetes in adults (LADA). LADA is a slow-developing autoimmune form of T1DM that usually occurs in people over 30 and typically presents without obesity. Individuals with LADA present with a less aggressive and gradual onset of ketosis without ketoacidosis. It is more common in males than females (ADA, 2023; Harding, 2020).
Educating patients about their risks and encouraging screenings can decrease the risk of developing T2DM and diabetes-related complications. Healthcare providers can offer education and support to decrease the risk associated with prediabetes and T2DM, including lifestyle management. There are modifiable and non-modifiable risk factors for prediabetes (ADA, 2023). Modifiable risk factors include:
- body mass index (BMI) greater than 25 kg/m2 (or greater than 23 kg/m2 in individuals of Asian descent)
- sedentary lifestyle (engaging in physical activity less than three times weekly)
- smoking
- HTN (BP greater than 130/80 or current treatment for HTN)
- high-density lipoprotein (HDL) cholesterol less than 35 mg/dL or triglyceride level less than 250 mg/dL (ADA, 2023; Brutsaert, 2022b)
Non-modifiable risk factors include:
- age (35 or older)
- ethnicity (non-Hispanic Black, Hispanic, Native American, Pacific Islander, or Alaska Native)
- first-degree relative with T2DM (mother, father, or sibling)
- personal history of hyperglycemia, gestational diabetes, or giving birth to a child who weighed more than nine pounds
- diagnosed with a disease associated with hyperglycemia, including polycystic ovarian syndrome (PCOS), HIV, fatty liver disease, or acanthosis nigricans (ADA, 2023; Brutsaert, 2022b)
The ADA recommends screening for risk factors in the general population (asymptomatic adult patients). Testing should be considered in those at risk, including those with a BMI of 25 kg/m2 or greater who also have one or more of the following risk factors: HTN, physical inactivity, history of CVD, HDL less than 35 mg/dL or a triglyceride level greater than 250 mg/dL, first-degree relative with diabetes, high-risk ethnicity (non-Hispanic Blacks, Pacific Islanders, Alaska Natives, Hispanics, Native Americans), individuals with a history of PCOS, and individuals with another condition associated with insulin resistance. Risk-based screening is recommended after age ten (or after the onset of puberty, whichever occurs first) in children who are overweight (BMI above 85th percentile) with at least one additional risk factor. The ADA risk test is a standard risk assessment tool available on the ADA’s website. Patients prescribed antipsychotic medications and those with prediabetes (impaired glucose tolerance [IGT], impaired fasting glucose [IFG], or a hemoglobin A1C of 5.7-6.4%) should be tested for diabetes annually. Urine glucose testing is minimally sensitive but can be used for screening before more invasive testing. Individuals with a history of gestational diabetes should be tested for T2DM at 4-12 weeks postpartum using the oral glucose tolerance test (OGTT) and at least every three years for the remainder of their life. Adult patients with average or low risk should begin screening at age 35. For abnormal screenings, a second test should be performed before initiating treatment. A second abnormal test confirms a diagnosis of prediabetes. Screening intervals are debated, but the ADA recommends that patients with normal results be retested at least every three years (ADA, 2023).
The pathophysiology of T2DM differs from T1DM based on the continued production of endogenous insulin by the pancreas. With T2DM, the insulin is either generated in insufficient quantities, used poorly by the tissues, or both. The most common risk factor for T2DM is obesity, especially abdominal adiposity (Harding, 2020). Genetic mutations that increase the risk of obesity and insulin resistance are found in individuals with T2DM. Twin studies have identified that when one twin has T2DM, the risk is approximately 3 in 4 for the other twin (ADA, n.d.-d). There are four significant metabolic abnormalities connected to the development of T2DM:
- insulin resistance, or the gradual decline in the typical reaction of skeletal muscle and adipose cells to insulin
- a decrease in the pancreas’s ability to produce insulin
- inappropriate glucose production by the liver
- altered production of hormones and cytokines by adipose tissue (Harding, 2020)
Risk factors for T2DM include prediabetes and the risk factors mentioned above. While T2DM is typically found in those over 45, there has been an increase in the incidence of T2DM amongst children, teens, and younger adults in recent years due to obesity and poor lifestyle choices, including inactivity. This is known as early-onset T2DM. While T1DM is almost entirely outside the individual's control, T2DM is influenced mainly by modifiable risk factors related to the individual's lifestyle choices. T2DM can be prevented or delayed with modifications to lifestyle, including exercising at least three times per week and a healthy diet based on complex, low GI carbohydrates (ADA, 2023; CDC, 2022c). Another risk factor for developing T2DM is metabolic syndrome. The individual diagnosed with metabolic syndrome has at least three of the following components:
- hyperglycemia (fasting plasma glucose [FPG] ≥ 100 mg/dL)
- abdominal obesity (abdominal circumference greater than 102 cm [40.8 inches] in men and 88 cm [35.2 inches] in women)
- HTN (≥ 135/85 mmHg)
- high triglycerides (≥ 150 mg/dL)
- decreased level of HDL (≤ 40 mg/dL in men and 50 mg/dL in women; Harding, 2020; Regufe et al., 2020)
Gestational diabetes (GDM) is similar to T2DM; however, this develops during pregnancy in individuals without a preexisting diagnosis of diabetes. Approximately 5.8% to 9.2% of all pregnancies in the US are affected by GDM. During pregnancy, insulin resistance may develop (or increase) due to the changes that occur, such as weight gain and hormone secretion from the placenta, leading to glucose intolerance and increased insulin requirements. All individuals have an increased need for insulin during late pregnancy, but those with GDM require more insulin throughout the entire pregnancy, necessitating treatment (US Preventive Services Task Force [USPSTF], 2021a). Risk factors include:
- previous pregnancy with GDM
- previous birth with a baby over nine pounds
- obesity
- over 25 years of age
- family history of T2DM
- PCOS
- ethnic backgrounds, including Native American, non-Hispanic Black, Hispanic, Alaska Native, Native Hawaiian, or Pacific Islander (CDC, 2022c)
Diabetes can occur if the ß-cells within the pancreas are damaged, injured, or destroyed due to other medical conditions or treatment for other diseases. Risk factors include Cushing’s syndrome, hyperthyroidism, recurrent pancreatitis, cystic fibrosis, hemochromatosis, and parenteral nutrition. Medications that can cause diabetes include corticosteroids, thiazides, phenytoin, and atypical antipsychotics such as clozapine (Clozaril). Diabetes caused by medications or an underlying condition may resolve once the underlying condition is treated or the medication is discontinued (Harding, 2020).
Presentation, Diagnosis, and Management of Diabetes
Prediabetes
Individuals are said to have prediabetes if they have IGT, IFG, or both. The ADA defines IGT as a BG level between 140 mg/dL to 199 mg/dL (7.8-11 mmol/L) two hours after ingesting a 75 g oral glucose solution (a two-hour OGTT). This type of test is primarily used in screening pregnant individuals but is also highly sensitive for T2DM. The patient should fast for at least eight hours before the OGTT. The ADA defines fasting as no caloric intake for at least eight hours. They define IFG as an FPG between 100 and 125 mg/dL (5.6-6.9 mmol/L). The World Health Organization (WHO) and others have a slightly lower limit for IFG (110-125 mg/dL or 6.1-6.9 mmol/L). The hemoglobin A1C (or simply A1C) indicates the percentage of the total hemoglobin with glucose attached to it in mmol/mol. A hemoglobin A1C greater than 5.7% but less than 6.4% indicates prediabetes. Prediabetes is not meant to be a diagnosis unto itself but indicates an increased risk of developing T2DM. Further, damage to blood vessels may already be occurring, yet this is often asymptomatic. This is a powerful rationale for individuals to have annual physicals with routine BG screenings (ADA, 2023).
Treatment/Management of Prediabetes
The goal of management is to prevent or delay the development of T2DM and the microvascular (e.g., retinopathy, nephropathy, and neuropathy) and macrovascular (e.g., atherosclerosis) complications related to diabetes. Evidence has proven that lifestyle changes such as increased physical activity and dietary modifications effectively decrease this risk in patients with prediabetes and control BG levels in patients with T2DM. The ADA suggests referral to an intensive behavioral lifestyle intervention program modeled on the CDC’s National DPP with an overall goal of weight loss (lose and then maintain a 7% to 10% reduction of initial body weight at a rate of two to three pounds per week) and routine physical activity (at least 150 min/week). This program allows for flexibility in selecting dietary and exercise options. The program focuses on decreasing calories, increasing exercise and physical activity, and maintaining healthy lifestyle behaviors along with social and psychological challenges that motivate the individual. The National DPP also assigns a trained coach to help the participants manage stress, stay motivated, and problem-solve to ensure success. Further goals toward preventing T2DM may include additional strength/resistance training and treating other CVD risk factors. These interventions increase the chance of avoiding the complications of prediabetes and subsequent T2DM. Those with confirmed prediabetes should be tested for T2DM annually per the ADA (ADA, 2023; CDC Diabetes Prevention Recognition Program, 2021; Jonas et al., 2021)
As previously mentioned, dietary management is a significant part of minimizing risk with prediabetes. The DPP suggests a reduction in total dietary fat and caloric intake. A specific diet is not recommended, but rather a varied diet that includes whole grains, nuts, berries, yogurt, coffee, and tea is associated with a reduced risk of diabetes. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and vegetarian diets have been linked to a reduced incidence of DM regardless of weight loss. Red meat and sugary beverages should be avoided, as well as high-carbohydrate diets (ADA, 2023; Benson & Hayes, 2020).
The APRN has many opportunities to promote success in patients with prediabetes through education, promotion of self-care, screenings, and encouraging continued monitoring by the healthcare team. One option for diabetic education is a program called Diabetes Self-Management Education and Support (DSMES), which is a service that helps patients diagnosed with prediabetes or T2DM learn how to manage their blood glucose, cholesterol levels, blood pressure, and how to care for themselves with the new diagnosis. DSMES participation can also prevent complications, reduce hospitalizations, and improve quality of life. Despite the effectiveness of DSMES in improving health outcomes, there is a participation rate of less than 7% of eligible patients within the first year of diagnosis (ADA, 2023; CDC, 2022e). Other diabetic education programs nationwide can be found on the Association of Diabetes Care and Education Specialists (ADCES) website. These programs strive to help patients prevent diabetes or, after being diagnosed, manage the day-to-day challenges of living with diabetes and support their management of long-term implications (ADCES, n.d.).
Metformin (Glucophage) is a biguanide, a group of medications that lowers glucose production in the liver and improves how the body utilizes insulin. The ADA recommends consideration of metformin (Glucophage) therapy for patients with prediabetes or those at a high risk to prevent or delay the onset of T2DM. The APRN should consider using this medication in patients aged 25-59 with a BMI above 35 kg/m2, those with an FPG of 110 mg/dL or above, those with an A1C of 6.0% or above, and individuals with a history of GDM. Metformin (Glucophage) is not approved by the US Food and Drug Administration (FDA) for this indication; however, it has the longest history of being a safe pharmacological option for T2DM prevention but is less effective than lifestyle modification and participation in a DPP. The recommended dose when using metformin (Glucophage) for T2DM prevention is 850 mg once or twice daily (ADA, 2023; Corcoran & Jacobs, 2022).
Type 1 Diabetes (T1DM)
Common signs and symptoms of T1DM include:
- polydipsia (excessive thirst as a result of increased serum osmolality)
- polyphagia (excessive eating due to increased hunger)
- polyuria (increased urination), especially nocturnal enuresis in children
- unintentional weight loss
- blurred vision or cataracts
- excessive fatigue/lassitude
- nausea, vomiting, or stomach pains
- perineal candidiasis
- DKA (Levitsky & Misra, 2023; Lucier & Weinstock, 2023)
The initial presentation of T1DM is DKA in approximately 30% of individuals but can range from 15% to 70%. The rate is higher for children under 6, those from a low socioeconomic background, unstable living conditions, or limited access to medical care. These patients may not realize they have diabetes until they have advanced symptoms requiring medical care (Gallagher & Siu, 2020; Levitsky & Misra, 2023). Symptoms of DKA may include:
- decreased alertness
- dry skin and mouth
- poor skin turgor
- flushed face
- hypotension
- tachycardia
- polydipsia/polyuria lasting for a day or more
- fruity-smelling breath
- headache
- muscle stiffness or aches
- nausea/vomiting or stomach pain
- Kussmaul breathing (Brutsaert, 2022c; El-Remessy, 2022)
In DKA, the liver breaks down body fat for energy due to the lack of glucose available for the cells, producing ketones as a byproduct. These ketones build up and cause metabolic acidosis. A compensatory symptom of Kussmaul respirations (deep and rapid breathing) may occur to compensate for the acidemia by reducing carbon dioxide. If untreated, DKA will lead to coma or death (Brutsaert, 2022c).
Diagnostic Tests for T1DM
According to the presentation of a patient with T1DM (e.g., emergency in DKA or office visit due to symptoms such as polyuria, polyphagia, polydipsia, and or weight loss), diagnostic tests may vary (Levitsky & Misra, 2023). The diagnostic criteria for diabetes are as follows:
- random plasma glucose of 200 mg/dL or more in a patient exhibiting classic signs of hyperglycemia
- this is the quickest option for testing when a patient presents with symptoms
- FPG of 126 mg/dL or greater after at least eight hours without caloric intake
- hemoglobin A1C of 6.5% (48 mmol/mol) or above
- two-hour OGTT BG 200 mg/dL or greater two hours after consuming 75 g glucose solution
- urine ketones: the presence of ketones suggests T1DM versus T2DM
- C-peptide levels typically mirror insulin levels; low levels of C-peptide and insulin can indicate T1DM
- antibody testing: Islet cell antibodies (ICAs) can be found in as many as 85% of patients with T1DM, and most also have antibodies to insulin (IAA), glutamic acid decarboxylase isoform 65 (GAD65), insulinoma antigen 2 (IA-2), and zinc transporter isoform 8 (ZnT8); IAAs are more commonly detected in children, and GAD65 is more commonly found in adults; the presence of two or more autoantibodies is an almost definite predictor of the development of diabetes (ADA, 2023; Levitsky & Misra, 2023; Lucier & Weinstock, 2023)
Unless there are clear clinical indicators of diabetes, repeat diagnostic testing is required to confirm the diagnosis. Repeat testing can be performed on the same or a new sample; however, if a new sample is used, it is recommended that repeat testing be completed as soon as possible. For patients exhibiting classic signs and symptoms of diabetes at the time of presentation, a random plasma glucose test is sufficient to confirm a diagnosis of diabetes (ADA, 2023).
Treatment/Management of T1DM
Hemoglobin A1C monitoring should be performed with any type of diabetes and prediabetes. The A1C is a blood test that provides an average of the BG level over the prior three months. While it is the most used test to diagnose prediabetes and diabetes, it can also determine the effectiveness of the treatment plan and if modifications should be made. The ADA recommends A1C monitoring at least twice yearly in patients with stable glycemic control and up to quarterly in those not meeting treatment goals or transitioning therapy strategies/modalities. Point-of-care testing provides more timely feedback for more efficient treatment changes; however, it is not accurate enough to be used for an initial diagnosis. Increased A1C levels are linked to increased diabetic complications such as retinopathy, nephropathy, and neuropathy (ADA, 2023).
A1C results are reported as an indirect estimate of average BG rather than mg/dL (displayed on glucometers). Table 1 indicates the A1C percentage as it correlates to the estimated average glucose (eAG), which the patient would see on their glucometer. Converting A1C to eAG can help facilitate patient communication and education around managing BG levels, as it is now in a unit that is better understood. The formula to convert A1C to eAG is 28.7 x A1C - 46.7 = eAG (ADA, n.d.-b).
Table 1
A1C Correlation with Estimated Average Glucose
A1C % | eAG mg/dL |
6 | 126 |
6.5 | 140 |
7 | 154 |
7.5 | 169 |
8 | 183 |
8.5 | 197 |
9 | 212 |
9.5 | 226 |
10 | 240 |
(ADA, n.d.-b)
The A1C may be inaccurately high or low due to kidney failure, liver disease, or severe hemolytic anemia. Certain medications can interfere with A1C levels, including opioids, some antiretrovirals, and medications that stimulate erythropoiesis. Blood loss or transfusions, early or late pregnancy, and certain blood disorders such as sickle cell anemia or thalassemia may all interfere with the accuracy of A1C results. Due to this variability in results, APRNs should use clinical judgment when assessing glycemic control using A1C alone (ADA, 2023).
A1C goals for nonpregnant adults are typically 7% or lower. In certain circumstances, a goal of less than 6.5% may be beneficial if achieved safely without significant hypoglycemia or other adverse effects. Personal goals may be adjusted due to age or medical conditions. Each patient’s A1C goal should be determined between the individual and their healthcare provider or team. For individuals who experience frequent hypoglycemia, a higher goal may be established to avoid severe lows. Younger people tend to have lower A1C goals since they have many years to live with diabetes. Some individuals may have higher goals due to multiple health conditions or to avoid hypoglycemic episodes. A goal of less than 8% may be acceptable in those patients with a history of hypoglycemia episodes (especially those who do not develop symptoms or cannot communicate the presence of symptoms), limited life expectancy, advanced macro- or microvascular complications, or extensive comorbid conditions (ADA, 2023; Brutsaert, 2022b).
In addition to the A1C goals, BG monitoring (called self-monitoring of blood glucose [SMBG]) should be performed throughout the day based on the insulin therapy utilized. Maintaining normal (70-120 mg/dL) BG levels minimizes vascular damage and future complications associated with diabetes (ADA, 2023). BG should typically be checked at the following times:
- upon awakening (or when fasting)
- preprandial (before a meal or snack)
- postprandial (two hours after a meal)
- at bedtime (ADA, 2023)
The ADA also recommends checking BG levels using SMBG before exercise, when the patient suspects hypoglycemia, after treating hypoglycemia (until euglycemic/normoglycemic again), and before and while performing important tasks (i.e., driving). Individual targets for BG will vary, but a preprandial goal is typically 80-130 mg/dL. The postprandial goal should be less than 180 mg/dL. The individual and their healthcare team will develop targets for the pre and postprandial BG levels as part of the treatment plan (ADA, 2023). Logging BG levels will allow the patient to discuss trends with their healthcare provider and determine opportunities for further improvement and management of their overall BG levels. Many new 'smart' glucometers perform this function automatically now, syncing their data with smartphone apps and websites to help visualize trends, including the time spent below, in, or above the BG range. The patient should note associated food intake and activity when logging BG levels within a paper log. Some apps allow the patient to enter this data directly into the program. The cost of testing supplies and medications is a concern for many people with diabetes. Although many companies provide glucometers at a low price, test strips and other needed accessories are sold at a considerable markup to increase profits. These can be very expensive, with short expiration periods and strict storage guidelines to maintain effectiveness, and are often not entirely covered by health insurance policies. The healthcare team should help connect the patient with resources that may offer low-cost or free supplies to ensure compliance (Mathew et al., 2023). The following outlines the proper steps that patients should follow when completing SMBG (Mathew et al., 2023):
- Gather and prepare all equipment needed.
- Ensure the glucometer is clean and adequately charged or has properly functioning batteries if applicable.
- Perform proper handwashing with soap and warm water for at least 30 seconds, massaging the hands to get blood into the intended finger. Dry well. Preferred testing sites include the second and third fingers.
- Remove a single test strip from the container and immediately reseal the container to ensure that the remaining test strips are not damaged by ambient moisture/humidity or extreme temperatures. Insert the test strip into the glucometer. Caution should be taken to ensure the tip with the sensor is not touched when handling the test strip.
- Prime the lancet if needed and then firmly apply the lancet to the site and press the trigger to release the needle and prick the finger, then squeeze from the base of the finger to extract a large drop of blood.
- Wipe away the first drop of blood with clean gauze, as this first drop could contain fluid that can affect results or be hemolyzed. Apply the second drop of blood onto the test strip by touching the test strip to the blood sample.
- Place the glucometer on a flat surface while the sample is being analyzed and clean the puncture site. The results will appear (in mg/dL) after a few seconds, depending on the specific glucometer being used and if any errors were detected (e.g., a sample that is too small or the machine times out). If not done automatically, this should be recorded immediately.
- The lancet and strip should be disposed of properly based on state and local medical waste and sharps regulations. The APRN should be sure to include in the patient education the importance of not sharing or reusing supplies such as lancets and test strips, even with family members.
The ADA recommends regularly evaluating patient technique when using a glucometer, especially in patients not meeting treatment goals. More education on technique and device use leads to better outcomes. Education can be completed in person or through online tutorials or training videos. They also caution that external factors such as extreme temperatures and patient factors such as oxygen saturation (monitors that use are glucose oxidase based), uric acid, ascorbic acid (vitamin C), galactose (milk sugar), xylose (a monosaccharide used in some foods as a sweetener), acetaminophen (Tylenol), and l-DOPA may affect glucometer accuracy (ADA, 2023).
Alternatively, continuous glucose monitors (CGM) measure interstitial glucose rather than plasma glucose and have recently become important in simplifying care for many patients with T1DM and some selected patients with T2DM. The units have improved in accuracy and affordability since their initial introduction to the market. They provide a readout called an ambulatory glucose profile (AGP) to assist patients and providers with data interpretation. They require additional patient education and initial training to ensure proper use. Many patients still use SMBG to calibrate their CGMs, confirm readings when discordant with symptoms, or when using an adjunctive CGM system (treatment decisions are based on SMBG). CGMs may help lower A1C levels and reduce hypoglycemia in T1DM patients with hypoglycemia unawareness or multiple episodes of hypoglycemia and those not meeting glycemic targets with SMBG. They should be considered and discussed in all children or adolescents with T1DM. CGMs can either be real-time (rtCGM, providing measurements of BG continuously with user alarms for preset BG thresholds or level changes) or intermittently scanned (isCGM, these measure BG continuously but only display and store results when prompted by scanning with a reader). Intermittent CGMs should be scanned often, at least every eight hours. The FDA has approved the FreeStyle Libre 2 (an isCGM), Dexcom G6, and FreeStyle Libre3 (both rtCGMs) to be integrated with other digital devices. Blinded CGMs may also be used by specialized clinics for brief periods (7-14 days) to temporarily analyze and correct patterns and trends of hypo and hyperglycemia but do not display results for the patient in real-time (ADA, 2023).
One of the more challenging aspects of managing diabetes is troubleshooting consistent irregularities. One common irregularity in T1DM patients is early morning hyperglycemia (before breakfast). While likely related to counter-regulatory hormones, two separate underlying mechanisms may be at play, leading to two disparate solutions. The dawn phenomenon refers to hyperglycemia that is not preceded by hypoglycemia and is related to the regular daily release of cortisol, growth hormone, and catecholamines (i.e., epinephrine, dopamine), occurring between 3 and 8 am in all individuals. This leads to the release of glucose from the liver and insulin resistance. To prevent this, patients should be counseled to increase the protein-to-carbohydrate ratio of the evening meal, consistently consume breakfast, and engage in increased physical activity in the evening. Providers may also consider adjusting the evening insulin dose to later (right before bed). The dawn phenomenon has been studied extensively and found to affect individuals of all ages with either T1DM or T2DM, with a prevalence of approximately 50% (O'Neal & Luther, 2023).
The Somogyi effect (or rebound effect/phenomenon) occurs after insulin doses are administered at bedtime, which causes hypoglycemia and triggers the release of counter-regulatory hormones (i.e., cortisol, glucagon, growth hormone, and epinephrine), which cause BG to increase for the same reason described above. If patients report consistent hyperglycemia upon waking and CGM is unavailable, they should be asked to check their BG for several days between 2 and 3 am. If their BG is consistently low at this time, this indicates that the Somogyi effect is the likely culprit. Providers should consider decreasing the patient’s evening insulin dose, adjusting the administration time to earlier, changing the type of insulin, or adding an additional bedtime snack. Bedtime insulin doses should be adjusted in small increments to avoid this phenomenon. If the patient’s BG is normal or elevated when checked between 2 and 3 am, it is more likely related to the dawn phenomenon. The Somogyi effect is highly debated in the healthcare community. Studies have shown that decreasing insulin dosing in the evening does not prevent morning hyperglycemia and that overnight hypoglycemia is more commonly associated with morning hypoglycemia, not hyperglycemia. No correlation has been made between daytime hyperglycemia and the levels of counterregulatory hormones found in the body (Reyhanoglu & Rehman, 2023).
The patient with T1DM is typically diagnosed by adolescence or young adulthood and will be impacted by the disease for their entire lifespan. Therefore, steps must be taken to avoid or delay the long-term complications commonly associated with diabetes. This can be accomplished by consistently maintaining BG levels within acceptable ranges. The poorly controlled diabetic is at heightened risk for micro and macro-vascular damage throughout the body. The APRN should help focus the patient’s efforts on preventing damage by carefully regulating BG levels. This is accomplished through eating a healthy, balanced diet focused on low-glycemic index (GI) foods and proper administration of insulin to meet the demands of dietary consumption. In addition, the T1DM patient should work with their healthcare team to individualize a treatment plan that works for their lifestyle, resources, and preferences (Brutsaert, 2022b; Wood & Peters, 2018). Additional details regarding the specific complications seen in patients with diabetes will be explored later in this module.
Diet/Carbohydrate Counting. While everyone should consume a healthy diet combined with an exercise regime, it is vital for people with diabetes. Proper nutritional intake, control of BG levels, and maintaining a healthy weight can decrease the impact of diabetes on the body. There is no diabetic diet, but the ADA does emphasize incorporating medical nutrition therapy (MNT) into the disease management plan. A varied diet consisting of the four food groups with a limited intake of empty carbohydrates high in added sugar, fats, and sodium is optimal; however, no universal eating plan works for all patients with diabetes. The combination, amount, and timing of meals or snacks, exercise, and medication all impact the BG level. Newly diagnosed diabetic patients often feel they cannot eat the foods they like or enjoy. The APRN must educate patients on eating smaller portions as part of a healthy diet that meets their nutritional needs and preferences. As previously mentioned, the National DPP, DASH, vegetarian, and Mediterranean diets have proven beneficial (ADA, 2023; Evert et al., 2019). The food groups that should be included in any healthy eating plan are:
- vegetables, including non-starchy and starchy options; suggested foods are carrots, greens, tomatoes, broccoli, carrots, squash, and peppers in the non-starchy group, and starchy options include potatoes and corn
- fruits, including bananas, grapes, oranges, apples, berries, and melons
- grains, primarily whole grains, including wheat, rice, oats, quinoa, cornmeal, and barley, as well as seeds
- protein, including lean meats, nuts, eggs, dried beans, tofu, and fish, such as tuna or salmon
- nonfat or low-fat dairy products, including yogurt, cheese, and milk
- heart-healthy fats, including avocados and oils that are liquid at room temperature, such as olive oil or canola oil (Evert et al., 2019; NIDDK, 2016)
Certain foods and drinks that should be avoided or severely limited in patients with diabetes include:
- fried foods
- high-sodium foods (limit should be less than 2,300 mg/day)
- high-sugar foods, including ice cream and candy
- high-sugar beverages, including juices, sweetened coffees, sports drinks, or soft drinks
- alcohol should be used in moderation (women should drink no more than one drink per day and men no more than two drinks per day), as it is high in glucose; Alcohol can cause delayed or nocturnal hypoglycemia and should be consumed cautiously in patients who use insulin or diabetes medications that increase the amount of insulin the body produces; alcohol consumption inhibits gluconeogenesis and can limit the individual's ability to identify the signs of hypoglycemia; food should be ingested simultaneously with alcohol to avoid hypoglycemia (Evert et al., 2019; NIDDK, 2016)
Most patients with diabetes should be taught to count carbohydrates by calculating the carbohydrate content of their meals and administering insulin based on the grams of carbohydrates consumed. Carbohydrates are converted into glucose after ingesting, and amounts can be found easily on nutritional facts and food labels. The patient should calculate their intake and match it with an appropriate insulin dose. Some individuals are given a set number of carbohydrates to consume and a dose of insulin to match. Other patients are given a range of carbohydrates that can be consumed and a ratio for calculating their insulin dose based on the number of carbohydrates eaten (e.g., 15 carbohydrates: 1 unit of insulin). A correction bolus can be given to bring BG within the desired range for the patient who continues to have an elevated BG even after their insulin bolus. Calculating carbohydrate intake and adjusting insulin bolus administration can be done with an insulin pump. An alternative to carbohydrate counting is the diabetes plate method, which teaches diabetic patients to use a 9-inch plate and split it into quarters. One-half (two-quarters) of the plate should be filled with non-starchy vegetables, one-quarter with protein, and the last quarter with carbohydrates. If the patient’s meal plan allows, they may eat a small piece/bowl of fresh fruit and drink a small glass of milk. This method applies best to lunch and dinner. This method helps many patients with portion size, but if additional teaching is required, many diabetic educators will correlate portions with everyday objects. Examples include comparing a serving of meat to the palm of the patient’s hand or a deck of cards, a serving of cheese to six dice, a serving (1/2 cup) of rice or pasta to a rounded handful or a tennis ball, one pancake or waffle to a DVD, and a serving of peanut butter (2 tbsp) to a ping-pong ball (ADA, 2023; Brutsaert, 2022b; Evert et al., 2019; NIDDK, 2016).
Some use the term GI or glycemic load (GL) in diabetic nutrition and evidence-based practice related to managing diabetes to rank foods based on how they influence postprandial glycemia. Food choices are ranked on how they compare to a specific reference food (i.e., white bread and glucose). High-GI foods (70-100, i.e., white bread, corn flakes, high fructose corn syrup, mashed potatoes, bagels, and waffles) will raise the individual’s BG level more than a food with medium (56-69, i.e., basmati rice, couscous, raisins, and cranberry juice) or low-GI foods (less than 55, i.e., legumes, fruits, starchy vegetables, whole grains). When meal plans are being developed, choosing low- or medium-GI foods is best. Occasionally eating high-GI foods can be offset by combining it with low-GI foods, allowing individuals to eat the foods they prefer while limiting the impact on their BG (Evert et al., 2019; Wood & Peters, 2018).
The GI of a particular food is affected by numerous factors, such as fat and fiber content, which typically lower the GI of a food. Other factors that impact the GI of food are the cooking or processing method. Also, the ripeness and storage of food may impact the GI. The riper the fruit, the higher the GI. The more cooked or processed a food is, the higher the GI, which is another rationale for eating whole foods that are less processed. The GI considers the type of carbohydrate in a food but not the quantity of the carbohydrate. Portion sizes are essential for both GI as well as weight management. Nutrition (protein, vitamin, and mineral content) should also be considered. Some nutritionally dense foods may have a high GI. A balance of all these aspects should be considered in order to provide optimal nutritional intake and support a steady BG level. While the GI of a particular food is not necessary to know to calculate the carbohydrate count, it is helpful to recognize that certain carbohydrates will affect the BG more than others (Wood & Peters, 2018). For further guidance on helping diabetic patients manage their nutrition, patients and care providers should explore the American Diabetic Associations’ website.
Exercise. Health and wellness are impacted by physical activity, as exercise improves BG control, well-being, cardiovascular fitness, muscle strength, and insulin sensitivity in diabetic patients and may lead to weight loss. Adults with T1DM or T2DM should exercise for at least 150 min/week at a moderate to vigorous intensity spread over three days with no more than two consecutive days without physical activity. Patients should be made aware of the variable effect of physical activity on their BG levels. Even mild exercise and physical activity can cause a decline in BG or hypoglycemia. Exercise can cause hypoglycemia through an increased need for glucose related to increased metabolic activities. This is especially true for individuals with T1DM. An individual’s BG will respond based on the BG level before starting the activity, the activity's intensity, the activity, the duration of the activity, the timing of the physical activity compared to meal times, and any modifications to insulin intake. A pre-exercise snack, frequent BG checks (before, during, and after exercise), and possible reduction in insulin should be encouraged to prevent hypoglycemia. The recommended target BG range before physical activity is 90 mg/dL to 250 mg/dL. The ADA recommends a pre-exercise snack with approximately 15 g of carbohydrates if the patient’s BG level is less than 90 mg/dL before exercising, especially if longer than 30 minutes of exercise is planned. Individuals with an insulin pump may be able to lower their basal rate during physical activity to avoid hypoglycemia. Patients should have an emergency management plan and travel with juice or glucose tabs while exercising away from home. In the event of severe hypoglycemia during exercise, the individual may need to ingest 5 to 15 g of sucrose during the workout. Timing, incorporating short sprints, and performing resistance exercises immediately before aerobic exercise are additional strategies to avoid hypoglycemia. Nocturnal hypoglycemia after exercise can be avoided by decreasing basal insulin, eating a bedtime snack, and using real-time CGM. This also applies to children and adolescents with diabetes, who may have unpredictable and more extended periods of activity. The ADA recommends that preschoolers, children, and adolescents engage in at least 60 minutes of physically active play daily. Parents should be counseled to monitor BG levels frequently and be prepared to give children snacks with 5-15 g of carbohydrates (depending on age/size) for every 30 minutes of active play (ADA, 2023; Brutsaert, 2022b; Colberg et al., 2016).
Occasionally, high-intensity exercise (i.e., high-intensity interval training [HIIT], sprinting, powerlifting) can also cause hyperglycemia due to elevated stress hormones in patients with diabetes, especially in those with T1DM. Reducing the insulin dosage before exercise may worsen this, as can consuming a high-carbohydrate meal before exercise. This risk can be mitigated by interspersing intense activity with periods of moderate-intensity aerobic exercise and a period of resistance training prior to aerobic training. If a patient’s BG is high (above 250 mg/dL) before exercise, they should utilize a home test to check their blood or urine for ketones. Exercise should be postponed or avoided if the ketone screen is positive (above 1.5 mmol/L). If there are no ketones in the blood/urine, they can proceed with mild to moderate exercise, with intense activity being delayed until BG drops to below 250 mg/dL. If BG is above 350 mg/dL before exercise and ketone testing is negative, insulin should be administered at 50% of the typical corrective dosing. Insulin administration should be completed before initiating physical activity. Hyperglycemia after exercise should be managed with a low-intensity aerobic cooldown or conservatively corrected with insulin (50% of a typical correction dose), as overcorrection increases the risk of nocturnal hypoglycemia. Efforts should be taken to develop consistent routines and understand the impact of insulin, food, and exercise on the individual’s BG levels. By recognizing this impact and working with the healthcare team, the best outcomes for exercise can be achieved. Devices such as CGMs can give even more precise information related to the impact of exercise on the body’s BG level over time. These devices are currently only recommended as an adjunct to SMBG during and after exercise due to inconsistent studies regarding their accuracy (ADA, 2023; Colberg et al., 2016).
The patient with T1DM should proceed cautiously when participating in high-risk activities such as scuba diving and skydiving. Patients with T1DM should obtain clearance from a clinician that specializes in diabetes and diving before scuba diving for the first time. These individuals are also limited to a maximum diving depth of 30 meters for no longer than 60 minutes. Additional precautions include diving with a slightly higher (rather than lower) BG level and diving with glucose gels (to be used if the BG level falls while submerged) and a partner aware of the patient’s diagnosis. Skydiving causes a release of adrenaline, which can increase BG levels. If an insulin pump or other diabetes device is used, care must be used to secure it during either activity (Jendle et al., 2020; Wood & Peters, 2018).
Pharmacological Treatment. Exogenous insulin will be needed for the patient with T1DM for life as no endogenous insulin is available; therefore, insulin therapy is the primary treatment modality. Daily needs for insulin and the type of insulin used varies in each patient based on illness, stressors, type and quantity of food intake, and activity level, amongst other factors. The goal of any exogenous insulin regimen is to mimic how the body releases endogenous insulin. Unfortunately, insulin cannot be absorbed orally and must be injected subcutaneously, infused intravenously, or inhaled into the lungs (Subramanian & Baidal, 2021). See Table 2 for examples of different types of insulins currently available.
Table 2
Insulins
Insulin | Onset | Peak | Duration | Teaching | |||
Ultra-rapid acting insulin | |||||||
aspart (Fiasp) - has added niacinamide | 2.5 minutes | 90-120 minutes | 5-6 hours | Can be administered at the start of a meal (or within 20 minutes of the first bite) | |||
lispro-aabc (Lymjev) | lispro-aabc is seen in the bloodstream within 1 minute |
|
| ||||
Rapid-acting insulin | |||||||
Glulisine (Apidra) | 5-15 minutes | 1 hour | 5 hours | Most can be mixed in a syringe with other insulins (do not mix Fiasp or Admelog); do not mix with other insulins in a pump. | |||
Lispro (Humalog, Admelog) | 15-30 minutes | 30-90 minutes | ≤ 5 hours | ||||
Aspart (NovoLog) | 15 minutes | 1-3 hours | 3-5 hours | ||||
Short-acting insulin | |||||||
Regular (Humulin R, Novolin R, Myxredlin, Velosulin R)
| 15-60 minutes | 2-4 hours | 6-12 hours | It can be mixed in the syringe with insulins other than Myxredlin; Humulin R in higher concentration (500 u/ml) should not be mixed and may last up to 24 hours. | |||
Intermediate-acting insulin | |||||||
NPH (Humulin N, Novolin N, ReliOn) | 1-1.5 hours | 4-12 hours | 10-24 hours | It can be mixed in the syringe with other insulins. | |||
Long-acting insulin | |||||||
Glargine (Optisulin, Lantus, Basaglar, Semglee) | 3-6 hours | No peak | 8-24 hours | CANNOT be mixed in the same syringe as any other insulins. | |||
Detemir (Levemir) | 3-4 hours | 3-9 hours | 24 hours | ||||
Ultra-long-acting insulin | |||||||
Degludec (Tresiba) | 1 hour | No peak | Up to 42 hours | CANNOT be mixed in the syringe with other insulins; Toujeo (glargine 300 u/ml) has a 6-hour onset and a 24-36-hour duration. | |||
Glargine U-300 (Toujeo) | 6 hours | No peak | Up to 36 hours | ||||
Intermediate/rapid combination | |||||||
Aspart protamine/aspart (Novolog Mix 70/30) | 10-20 minutes | 1.8-3.6 hours | 6-24 hours | CANNOT be mixed in the syringe with other insulins; Lispro combinations have a shorter duration than aspart. | |||
Lispro protamine/lispro (Humalog Mix 50/50 or 75/25) | 15 minutes | 30 minutes to 4 hours | 11-22 hours | ||||
Intermediate/short combination | |||||||
NPH/regular (Humulin 70/30, Novolin 70/30) | 30 minutes | 2-10 hours | 18-24 hours | CANNOT be mixed in the syringe with other insulins. | |||
(Subramanian & Baidal, 2021; Woods, 2023)
As seen in Table 2, insulin can be premixed in various combinations for convenience or for individuals who have difficulty drawing insulin from two bottles. This can be helpful to older adults or those suffering from limited eyesight or manual dexterity. Combination insulin also delivers two types of insulin while only requiring one injection and is more cost effective than purchasing two different types of insulin. Insulin pens have a prefilled cartridge and attachment site for a needle. The pen is typically constructed with a dial to adjust the dosage of insulin delivered. Insulin pens contain only a single type of insulin, eliminating the possibility of mixing two types of insulin into a single injection. Insulin via a dry powdered inhaler, insulin human (Afrezza), has been introduced as an alternative to injections. This insulin is introduced via the lungs and absorbed into the bloodstream within seconds. This insulin advertises an onset of 12 minutes, peaks within 30-60 minutes, and lasts up to 4.5 hours. The dosing of insulin human (Afrezza) is not adjustable as it is only available in 4, 8, and 12-unit cartridges. Due to the administration method, insulin human (Afrezza) is contraindicated in patients with chronic lung diseases. Patient education for insulin administration should include information on the insulin regime, including the type of insulin, times of administration, methods of administration, and adverse reactions to monitor for. Patients should be educated on site rotation to preserve subcutaneous tissue integrity. The injection site affects insulin absorption speed; abdominal injections are absorbed the fastest, followed by the upper arm and, finally, the thighs/buttocks. The APRN should encourage patients to vary the exact location but to be consistent regarding using the same area of the body each day/time. Patients should be educated according to the insulin's strength, which may vary. While the standard and most common insulin strength is U-100 (100 units per mL), there are U-300 (glargine U-300 [Toujeo]) and U-500 (Humulin R U-500) options available. The older version (U-40) is no longer common other than in veterinary medicine, but many syringes still include these measurement markings (ADA, 2023; Subramanian & Baidal, 2021; Woods, 2023).
Most patients with T1DM are maintained on a basal/bolus dosing regimen. A basal dose of insulin will deliver continuous BG control. This can be attained via long-acting or ultra-long-acting insulin or via an insulin pump with rapid-acting insulin continuously administered in small doses. The basal dose is typically administered at the same time every day and does not change with increased BG levels; it is intended to mimic the normal pancreas in a healthy individual, which constantly secretes a basal dose of insulin to manage BG levels. A bolus insulin dose is administered throughout the day (i.e., with meals) to counteract food intake or elevated BG levels (Brutsaert, 2022b). The ADA recommends taking regular insulin (Humulin R, Novolin R) approximately 30 minutes before eating to optimize its effectiveness in relation to the glucose influx in the blood. The Diabetes Control and Complications Trial (DCCT) provided evidence that intensive therapy (INT), including multiple daily injections (MDIs) per day or continuous subcutaneous insulin infusion (CSII), provided optimal glycemic control, lowered A1C, and improved long-term outcomes for T1DM patients, even as many as 30 years after the start of the original trial. INT achieves an estimated 50% reduction in macrovascular and microvascular damage and the resulting retinopathy, nephropathy, and neuropathy. The primary adverse effect reported during the initial study included severe hypoglycemia (ADA, 2023)
CSII, or insulin pump therapy, is a convenient way for patients with T1DM to reduce the insulin injections required to manage their blood sugar. This administration method also more closely mimics the physiologic way that insulin is secreted naturally. Computerized pumps deliver insulin into the subcutaneous tissue via a catheter inserted and then taped into place (an infusion set). They are programmed to deliver a basal rate and allow for bolus dosing per the user's input at mealtimes or in response to elevated BG levels as needed. Most patients report higher satisfaction and improved quality of life when using CSII compared to MDI; however, pumps may be complicated for technologically challenged patients, and most cannot be worn while swimming, which may be problematic for pediatric patients during the summertime. An insulin pump should be considered in patients with T1DM who are interested in this form of management, are very active, have frequent hypoglycemia episodes, those with gastroparesis (delayed gastric motility and absorption), and those planning to become pregnant. Some insulin pumps are designed to communicate with and respond to compatible CGMs (see prior discussion on FDA-approved devices for this purpose). The combination of real-time CGM and a compatible insulin pump is called a sensor-augmented pump (SAP), and studies thus far indicate that this may improve glycemic variability and reduce the rate of hypoglycemia in patients with T1DM. Unfortunately, pediatric and adolescent populations have high rates of noncompliance with SAP, reducing its effectiveness significantly in this group of patients. The FDA has also approved many closed-loop systems which use an algorithm to determine when to administer insulin based on real-time glucose levels. These devices have demonstrated effectiveness in adolescents and adults with T1DM. The International Diabetes Closed Loop (iDCL) trial found that over 6-months utilizing a closed-loop system led to significantly increased time spent within the target glycemic range, decreased A1C, and reduced episodes of hypoglycemia when compared to an SAP (ADA, n.d.-c, 2023; Janez et al., 2020).
Another treatment option for diabetes is pramlintide (Symlin), an injectable synthetic hormone based on amylin. This naturally occurring neuroendocrine hormone is released into the bloodstream, like insulin, after a meal. Patients with diabetes are deficient in amylin. Pramlintide (Symlin) delays gastric emptying, blunts pancreatic secretion of glucagon, and promotes a feeling of fullness, reducing appetite and caloric intake. The FDA has approved this drug for treating T1DM and T2DM in conjunction with insulin to be given via subcutaneous injection with meals. Further positive outcomes seen with pramlintide (Symlin) include weight loss of approximately 1 kg, a reduction in A1C by 0.3% to 0.4%, and decreased insulin requirement of up to 50% (ADA, 2023; Woods, 2023).
Surgical Management of T1DM. There currently exists one curative option for T1DM: a pancreas transplant. There are two types of pancreatic transplants: whole pancreas and islet cell. Many patients have experienced positive results from a pancreas transplant, while others have not had the same success. This variability has prevented the procedure from being utilized more commonly; it is currently reserved for those with significant complications related to T1DM, frequent and severe episodes of hypoglycemia (more than two episodes in the previous 24 months) or DKA, and a poor quality of life despite treatment with insulin. Patient outcomes have improved alongside surgical technique, the effectiveness of immunosuppression drugs, and the selection of donors. In patients with advanced kidney disease, the pancreas transplant is often combined with a kidney transplant from the same donor, known as a simultaneous pancreas and kidney transplant (SPK). A pancreatic transplant using a different donor may be performed after a kidney transplant. SPK is the treatment of choice for individuals with T1DM and end-stage renal disease (ESRD) and is also used in patients with T2DM and ESRD. About 10% of all pancreas transplants are done in those with T2DM who have a combination of low insulin production and resistance, unstable glycemic control, and low perception of hypoglycemia symptoms. The primary benefit of a pancreas transplant is the ability to maintain euglycemia, a normal glucose concentration in the blood, without taking exogenous insulin. The long-term damages caused by diabetes are prevented or delayed, and nerve damage from diabetes is slowed or even reversed after a transplant. Surgical risks include blood clots, infection, bleeding, and urinary complications. The primary risk of a pancreas transplant is the body's rejection of the foreign organ and the requisite immunosuppressant drugs that must be taken to avoid such a rejection. While immunosuppressant medications are necessary to lower the chance of rejection, these drugs increase the risk of infections, cancer, and opportunistic diseases. Other side effects include osteoporosis, hypercholesterolemia, HTN, gastrointestinal symptoms, sensitivity to light, weight gain, acne, swollen gums, and hair growth or loss. Pancreas transplants require a higher dose of immunosuppressant drugs due to the increased immunogenicity of the organ, increasing the risk of adverse effects. In patients that have undergone a pancreas transplant, signs and symptoms of rejection include abdominal pain/increased tenderness at the transplant site, fever, hyperglycemia, vomiting, and oliguria (decreased urination). The diet and lifestyle recommendations described above should be continued in transplant recipients to ensure long-term health and well-being (Bahar & Devulapally, 2023; Kochar & Jain, 2021).
Due to the high risk of rejection of the pancreas, there has been research on and success with islet transplants. Islet cells, which produce insulin, are destroyed in T1DM. Only 1-2% of the pancreas comprises islet cells. Hence, the transplantation of islet cells conveys significantly less risk of rejection, less surgical risk, fewer postoperative complications, and decreased cost. Islet cell transplantation may also be an option for pediatric patients. During the procedure, islet cells are taken from a donor pancreas and injected into the recipient's portal vein, possibly repeatedly. The new islet cells should start producing insulin gradually and thus reduce or eliminate the need for exogenous insulin. Very close monitoring of the BG in the initial phase of transplantation is essential to maintain euglycemia. Islet transplantation has been limited to healthcare centers that participate in clinical research in part due to a decision made by the FDA in 1993 to categorize allogeneic islet cells as biologics instead of solid organs. In 2021, the FDA voted to endorse the biologic donislecel (Lantidra) for use in patients with brittle T1DM that are not adequately managed with available treatments. Recipients reported improved quality of life and better overall health following the administration of this cellular suspension of allogeneic pancreatic islet cells. Procedural risks include rejection and transplant failure; otherwise, risks are limited to pain, bleeding, and blood clots. Currently, immunosuppressant drugs are still needed to avoid rejection, but research is ongoing to potentially eliminate that need by using macro- or microencapsulation of the islet cells. One primate study showed that administering apoptotic donor splenocytes with immunosuppression for approximately two weeks induced self-tolerance without rejection one year after transplantation. There is also ongoing research on using porcine islet cells as an alternative to donor islet cells (Bellin & Dunn, 2020; Mutanga et al., 2023; Pullen, 2021).
Type 2 Diabetes (T2DM)
Due to its slow, insidious onset, T2DM is often silent and without symptoms for years before diagnosis. Like patients with prediabetes, these individuals may be unaware of a problem until they have been exposed to abnormally elevated BG levels for extended periods and begin to experience complications. T2DM is often discovered on a routine check-up, annual physical, pre-employment screening, or when the patient develops a wound that will not heal, repeated vaginal infections, or other infections of increased frequency. Further presenting symptoms may be blurred vision, polyuria, polydipsia, polyphagia, numbness or tingling of the hands or feet, or dry skin. Adults starting at age 35 and individuals with obesity and at least one additional risk factor should be screened at routine intervals (typically every three years or sooner if risk factors change) for early intervention and recognition of the disease (ADA, 2023 Brutsaert, 2022b).
Diagnostic Tests for T2DM
The diagnostic criteria for T2DM mirror those listed above for T1DM; the exceptions are that ketone testing and antibody testing are not typically applicable in T2DM patients, although antibody and c-peptide testing may be indicated to differentiate between T1DM and T2DM. High C-peptide levels may indicate T2DM; however, this test should not be performed until hyperglycemia has been resolved, as high C-peptide can be suppressed due to glucose toxicity. Following abnormal screenings of BG or A1C, a second test is typically performed (may be repeated on the same sample) prior to confirming the diagnosis of T2DM. When diagnosing T2DM, other historical data and symptoms should be considered (ADA, 2023; Levitsky & Misra, 2023).
Treatment/Management of T2DM
The patient primarily manages T2DM daily with education, training, and support from the healthcare team. Management includes eating a healthy diet, physical activity at least three times per week as described previously, and, if needed, SMBG and administering medication (Brutsaert, 2022b;).
The initial diagnosis of T2DM should prompt a referral to other healthcare team members, including a registered dietician, diabetes educator, exercise specialist, and mental health provider, as appropriate. Other referrals may include an ophthalmologist, dentist, podiatrist, and potentially a bariatric doctor for patients also diagnosed with obesity (BMI above 40 kg/m2). The entire family and any direct caregivers should be involved in diabetic education. Long and short-term goals should be established with the primary and specialty healthcare provider, dietician, and diabetic educator, taking into consideration the patient's preferences, personal goals and priorities, current lifestyle habits, clinical characteristics, and barriers such as cognitive deficits, motivation, monetary constraints, and other social determinants of health. Goals should be SMART (specific, measurable, achievable, realistic, and time-limited), reviewed at each subsequent visit, and updated as appropriate. If applicable, the healthcare team must discuss and support smoking cessation. Comorbidities such as HTN and hypercholesterolemia must be adequately managed in patients with T2DM. Vaccinations should be kept up-to-date (based on age-related recommendations) and include hepatitis B, influenza, COVID-19, and pneumococcal pneumonia vaccines since diabetes impairs immunity. Stress as a result of diabetes is known as diabetic distress and affects 18-45% of individuals with T2DM. Diabetes distress can lead to decreased medication adherence, elevated A1C levels, and poor eating and exercise habits. Diabetes distress should be managed with additional education, behavioral intervention, regular exercise participation, adequate sleep, and relaxation techniques such as meditation and yoga. These strategies can help improve overall health and well-being in patients with T2DM. Patients with T2DM need to be active in their treatment plan and encouraged to discuss concerns with their healthcare team (ADA, 2023; Brutsaert, 2022b; Goyal & Jialal, 2023).
Providers should seek out additional resources for patient education, such as the DSMES toolkit found on the CDC website. This program provides diabetic education and support to patients and their families and is often eligible for reimbursement by Medicare, most state Medicaid agencies, and many private insurers. Medicare Part B members are eligible for 10 hours of diabetes education during their first year after diagnosis, followed by two additional hours of education every year following. For reimbursement purposes, the service must be coded under diabetes self-management training (DSMT). In 2020, there were 2,158 sites providing DSMES services nationwide, with approximately 1 million individuals participating. Details regarding locally recognized/accredited programs can be found on the CDC, ADA, and ADCES websites. Less than 5% of Medicare patients and 6.8% of privately insured patients with diabetes have participated, even though studies indicate that DSMES positively impacts lifestyle changes, decreases A1C levels, prevents or delays complications, improves quality of life, and reduces hospitalizations. Unfortunately, access continues to be an issue; accredited programs are located in 56% of counties nationwide, and 62% of rural counties have limited access (CDC, 2022d).
SMBG may provide limited clinical benefit (it does not significantly reduce A1C levels in studies) in those T2DM patients not using insulin. For some of these patients, SMBG may provide valuable insight into the effect of diet, exercise, and medication on BG levels and be helpful while adjusting diet and exercise or other medications (especially those that may cause hypoglycemia). Patients with T2DM who do not require INT and can be maintained on basal insulin with or without oral medications may achieve lower A1C levels with SMBG (especially when assessing fasting BG levels to inform dose adjustments). Patients with T2DM on INT should abide by the same guidelines for SMBG or CGM, as described above for T1DM patients, with minor modifications to customize for each patient. When appropriately used, CGM may reduce A1C levels and episodes of hypoglycemia in T2DM patients on insulin who are not meeting glycemic targets. Like T1DM, most T2DM patients should have an A1C goal of less than 7%, including children and adolescents. A goal of less than 6.5% may be appropriate if it can be achieved without significant hypoglycemia. A goal of 7.5% may be necessary if the risk of hypoglycemia is increased (ADA, 2023).
Pharmacological Treatment. Many patients with T2DM can avoid oral and subcutaneous hypoglycemic medications or insulin with changes to diet, exercise, and the other lifestyle modifications discussed above. In other patients, these measures are inadequate, and medications are added when needed to avoid complications related to consistently elevated BG levels. Oral medications to lower BG levels are typically the first-line pharmacological treatment for T2DM (see Table 3); patients must understand that these medications work best when combined with dietary changes and increased physical activity. Metformin (Glucophage) is typically the first medication prescribed for T2DM and sometimes for prediabetes in individuals at a high risk of developing T2DM. It decreases A1C, weight (marginally), and cardiovascular risk; however, as previously discussed, it has not been approved for this purpose. This is especially appropriate in treating metabolically stable patients with A1C levels under 8.5% and renal function above 30 mL/min/1.73m2. Metformin (Glucophage) is also commonly used in individuals with PCOS to induce ovulation; this medication should be stopped by the end of the first trimester once pregnancy is confirmed. Young patients with T2DM and BG levels above 250 mg/dL and an AIC level above 8.5% who are symptomatic (polydipsia, polyuria, nocturia, and weight loss) should be started on basal insulin while metformin (Glucophage) is titrated (Brutsaert, 2022d; Woods, 2023).
When metformin (Glucophage), diet, and exercise are not successful in controlling BG levels, another oral or injectable medication should be added. Sulfonylureas and meglitinides can cause hypoglycemia, and patients should be educated regarding the signs and symptoms of hypoglycemia when prescribed these medications. Thiazolidinediones are typically not the first-choice treatment due to their side effect profile. They are used only in unique cases where BG control is not attained with other drug categories. Dipeptidyl-peptidase 4 (DPP-4) inhibitors often have an intermediate effect on BG levels and a neutral effect on weight. Sodium-glucose transporter 2 (SGLT2) inhibitors may reduce the risk of acute myocardial infarction or stroke and are recommended in patients with atherosclerotic cardiovascular disease (ASCVD) or heart failure, elevated cardiovascular risk, or kidney disease (Brutsaert, 2022d; Woods, 2023).
- Table 3
- Oral Antidiabetic Medications
Medication Names | Mechanism of Action | Advantages | Potential Side Effects |
Biguanides
| Enhances insulin sensitivity and decreases glucose production and absorption | Effective, has the potential for minor weight loss, low cost | Nausea, diarrhea, vitamin B12 deficiency, rare lactic acid buildup if used in patients with kidney or liver failure |
Sulfonylureas
| It works by inducing the pancreas to secrete more insulin | Effective, low cost, and longer duration than meglitinides | Hypoglycemia, weight gain, and skin rash; may increase insulin resistance over time, leading to decreased effectiveness |
Meglitinides
| It works by inducing the pancreas to secrete more insulin | Faster onset than sulfonylureas | Hypoglycemia, weight gain, interacts with alcohol and causes nausea and vomiting |
Thiazolidinediones
| Enhances insulin sensitivity in tissues, but the specific mechanism of action is not understood | May increase HDL cholesterol | Weight gain (over 10 kg), peripheral edema, increased risk of heart failure and fractures; rosiglitazone (Avandia) may increase the risk of myocardial infarction, stroke, and anemia; pioglitazone (Actos) may increase the risk of bladder cancer |
Alpha-glucosidase inhibitors
| Inhibit the enzymes in the small intestine that hydrolyze carbohydrates decreasing the absorption rate | Are safe and can be used in combination with other oral antidiabetic medications and insulin | Flatulence, dyspepsia, diarrhea, and abdominal pain; patients usually discontinue treatment due to these side effects |
DPP-4 inhibitors
| Increases insulin and decreases glucagon production | Do not cause hypoglycemia or weight gain | Headache, sore throat, upper respiratory infections, joint pain, increased risk of pancreatitis |
SGLT2 inhibitors
| It prevents the kidneys from reabsorbing sugar back into the blood, excreting it via the urine | May lower BP and reduce the risk of acute myocardial infarction or stroke; could promote weight loss | Urinary tract infection (UTI), yeast infections, rare genital infections, hypotension, and increased risk of DKA; canagliflozin (Invokana) increased risk of lower limb amputation |
(Brutsaert, 2022d; Woods, 2023)
Not all individuals can control their BG with oral medications. GLP-1 receptor agonists are non-insulin injectable medications that slow down digestion and thus decrease BG levels. They are also recommended by the ADA (2023) for treating T2DM in those with ASCVD or heart failure, elevated cardiovascular risk, or kidney disease. These medications are preferred over insulin in patients with T2DM whenever possible. They may reduce BP and promote weight loss but have potential gastrointestinal adverse effects such as nausea, vomiting, constipation, abdominal distention, and diarrhea; however, these effects are typically temporary and can be decreased with changes in diet, such as decreased meal size and avoidance of spicy foods. Additional side effects include headache, fatigue, and dizziness. There is also an increased risk of pancreatitis when taking these medications. Due to the development of thyroid adenomas and carcinomas in rodents, there is a black box warning addressing the use of GLP-1 receptor agonists in patients with a personal or family history of medullary thyroid carcinoma. There are various formulations, and dosing may be twice daily, daily, or weekly, with all administered subcutaneously. Examples are exenatide (Byetta, Bydureon), dulaglutide (Trulicity), semaglutide (Ozempic), lixisenatide (Adlyxin), albiglutide (Tanzeum), and liraglutide (Victoza). Semaglutide is also available for oral administration under the brand name Rybelsus. Liraglutide (Victoza) and semaglutide (Ozempic) have been associated with a decreased risk of acute myocardial infarction and stroke in patients at increased risk. The ADA considers semaglutide (Ozempic) and high-dose dulaglutide (Trulicity) to have very high efficacy for lowering glucose. The GLP-1 receptor agonist liraglutide (Victoza) can be used in patients with T2DM over ten who are not meeting glycemic targets on metformin (Glucophage) and lifestyle interventions and is also considered highly effective for weight loss and lowering glucose (ADA, 2023; Brutsaert, 2022d).
A new class of medication is the dual incretin agonist composed of a glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptor agonist. The first drug in this class is tirzepatide (Mounjaro). This medication works by increasing insulin secretion, decreasing glucagon, and slowing gastric emptying, which decreases appetite and promotes weight loss. It is administered subcutaneously once weekly. The adverse effects and contraindications of tirzepatide (Mounjaro) are the same as those for GLP-1 receptor agonists used alone. Treatment with tirzepatide (Mounjaro) has been shown to decrease the progression of prediabetes to diabetes. The ADA has deemed this medication to have very high efficacy for lowering glucose (ADA, 2023; Brutsaert, 2022d).
Insulin should be added to the treatment plan when BG levels are not adequately controlled with lifestyle modifications, oral medications, or non-insulin injectable medications. Initially, a single injection of long-acting insulin such as glargine (Lantus) or detemir (Levemir) may be combined with other oral or subcutaneous medications. When insulin is used, the ADA (2023) recommends combining it with a GLP-1 receptor agonist to enhance efficacy. Liraglutide is available as a combined product with once-daily dosing of insulin degludec (Xultophy 100/3.6), and insulin glargine and lixisenatide are available in a combined product (Soliqua 100/33). Some patients with T2DM eventually worsen and require multiple injections of insulin daily. Other medications that may be required for T2DM patients include antihypertensive medications to control blood pressure (BP) and protect renal function, low-dose aspirin to reduce cardiovascular risk, and cholesterol-lowering medications to manage hypercholesterolemia (ADA, 2023; Brutsaert, 2022d).
Weight Loss. Diet, exercise, and behavioral therapy is the recommended method to reduce body weight in patients with T2DM and a BMI above 25 kg/m2. It should be explained to patients that losing at least 5% of their body weight will benefit BG control and reduce CV risk factors and complications. Intensive behavioral intervention sessions (at least 16 sessions over six months) focusing on dietary changes with a goal of a 500-750 calorie daily deficit, increased physical activity, and behavioral adjustments should be included in the plan of care alongside medical interventions to support lifestyle changes leading to successful weight loss. Motivation and willingness to lose weight should be assessed first, as interventions are unsuccessful in patients who are not eager to lose weight. Diets should be individualized based on personal taste, cultural factors, food availability, and macronutrient needs based on activity level and lifestyle. Weight maintenance programs are recommended to help patients sustain their short-term goals for over one year by incorporating monthly support and weekly weight assessments (ADA, 2023).
Patients with a BMI over 27 kg/m2 who cannot achieve their desired weight loss goals with diet, exercise, and behavioral therapy may benefit from pharmacological agents approved by the FDA for weight loss, which have been shown to lead to improved glycemic control in T2DM patients. Phentermine (Adipex-P, Lomaira) is only approved for short-term (less than 12 weeks) use. The typical adult dose is 8-37.5 mg once daily. Phentermine, when combined with topiramate (Qsymia), is approved for use longer than 12 weeks. Orlistat (Xenical, Alli) 60 mg is available over-the-counter or prescription for the 120 mg formulation. It works by inhibiting the action of lipase and thus reducing the absorption of dietary fat intake. Due to the mechanism of action, typical side effects include flatulence and bowel urgency. Naltrexone/bupropion (Contrave) combines an opioid antagonist with an antidepressant that blocks norepinephrine and dopamine reuptake. This medication has a black box warning for increased risk of suicidal ideation or behavior due to the antidepressant. Semaglutide (Ozembic, Wegovy) is considered highly effective for weight loss and is used as an adjunct to diet and exercise. It is administered subcutaneously once weekly or orally once daily. Semaglutide (Ozembic) should be used cautiously with insulin or insulin secretagogues such as glipizide (Amaryl) due to the increased risk of hypoglycemia. The ADA has deemed Tirzepatide (Mounjaro) to have very high efficacy for weight loss. Liraglutide (Saxenda, Victoza) and high-dose dulaglutide (Trulicity) are considered highly effective for weight loss (ADA, 2023; Woods, 2023)
There are also medical devices FDA-approved for short-term weight loss. These include gastric balloon implantation, vagus nerve stimulator, or gastric aspiration therapy. Despite the availability of these devices, they are rarely utilized for weight loss in diabetes due to the high cost and lack of coverage by health insurance providers. The FDA has approved an oral pharmacological treatment hydrogel (Plenity) for long-term use in patients with a BMI greater than 25 kg/m2. This drug mimics the effects of an implantable gastric band. The patient takes the hydrogel (Plenity) with water 30 minutes before eating. Once the water mixes with the hydrogel (Plenity), it begins to expand within the stomach to decrease the available space for food and create a feeling of being full, leading to decreased calorie intake (ADA, 2023).
Individuals with T2DM with a BMI over 40 kg/m2 (over 37.5 kg/m2 in Asian Americans) may be referred for weight loss (bariatric) surgery. Adults with a BMI between 35 and 39 kg/m2 (32.5-37.4 kg/m2 in Asian Americans) could also be considered for weight loss surgery if they are unable to lose weight and decrease their medical comorbidities (including hyperglycemia) with diet, exercise, behavioral counseling, and pharmacological methods. BG levels are often drastically improved by the weight loss that occurs after bariatric surgery. The healthcare team must discuss risks such as long-term nutritional deficiencies, osteoporosis, and death with the patient (ADA, 2023; Woods, 2023).
Gestational Diabetes (GDM)
Patients with GDM are often asymptomatic. Ideally, patients with risk factors or part of a high-risk population planning on becoming pregnant should be screened for undiagnosed prediabetes or T2DM before conception. If preconception screening is not completed, universal screening before 15 weeks’ gestation should be considered to differentiate between GDM and diabetes complicated by pregnancy. Pregnant individuals are screened in the US between 24-28 weeks if early screening is negative or not completed. Two strategies are used to test for GDM: the one-step or the older two-step approaches (ADA, 2023; Goyal & Jialal, 2023). Both of these tests are intended for pregnant individuals without a prior history of diabetes (ADA, 2023).
- One-step strategy (derived from the International Association of the Diabetes and Pregnancy Study Groups [IADPSG] and currently recommended by the ADA)
- After fasting overnight, an FPG is drawn before starting the test. A 75 g glucose solution is ingested, and then the patient’s BG is checked one and two hours post-ingestion.
- If any of the three BG levels exceed the established threshold, the patient is diagnosed with GDM. The fasting threshold is 92 mg/dL, the one-hour threshold is 180 mg/dL, and the two-hour threshold is 153 mg/dL.
- Two-step strategy (recommended initially by an NIH panel in 2013 and currently by the American College of Obstetricians and Gynecologists [ACOG])
- Without fasting, the patient drinks a 50 g glucose solution with a BG level check one hour later. If the BG is higher than 130-40 mg/dL (depending on the professional organization making the recommendation) at one hour, it indicates a need for additional testing.
- The second step is indicated only in individuals with BG above 130-140 mg/dL level in step #1. Like the one-step strategy, the pregnant patient fasts overnight and presents for an FPG. The patient then drinks a 100 g glucose solution, and BG checks are done hourly for three hours. Two of the four BG readings that are abnormally high constitute a positive test (95 mg/dL fasting, 180 mg/dL at one hour, 155 mg/dL at two hours, and 140 mg/dL at three hours).
- ACOG states that even one elevated BG result is enough to diagnose GDM.
Treatment/Management of GDM
Primary care providers should educate non-pregnant individuals of childbearing age about the importance of weight loss and regular exercise to reduce their risk of developing GDM. Pregnant individuals with diabetes (GDM or pre-existing) should be instructed to check their BG routinely; fasting and postprandial BG checks are recommended. Per ACOG recommendations, fasting BGs should be below 95 mg/dL, and postprandial BGs should be below 140 mg/dL at one hour, and below 120 mg/dL at two hours. The ADA recommends very similar targets for pregnant individuals with T1DM or T2DM. Per the ADA, fasting glucose should be 70-95 mg/dL, one-hour postprandial 110-140 mg/dL, and two-hour postprandial 100-120 mg/dL. Alternately, CGM may be utilized in addition to BG monitoring to improve A1C levels and neonatal outcomes, especially in pregnant individuals with T1DM. In any individual, the A1C is lower during pregnancy. Due to this, the A1C target during pregnancy is less than 6% but may be increased to 7% to avoid hypoglycemia. Lifestyle modification, including diet (based on referrals to a nutritionist/dietary consult) and exercise, is recommended for treating diabetes of any type during pregnancy. Weight loss during pregnancy is not encouraged. Insulin is the first-line pharmacological therapy for GDM if lifestyle modifications do not achieve glycemic targets. It is also the first-line treatment in pregnant individuals with pre-existing T2DM or T1DM. Metformin (Glucophage) and glyburide (DiaBeta) are considered safe secondary alternatives, but patients should be warned that both medications cross the placenta to the fetus. Other oral/injectable medications currently lack long-term safety data in pregnant patients and should be avoided. Close monitoring of the fetus for optimal growth and development is required. Following delivery, insulin requirements quickly decrease to roughly 50% of previous requirements, and the patient and care team should be cautious to avoid hypoglycemia immediately postpartum. To reduce the risk of developing GDM in future pregnancies, the patient should be encouraged to lose weight and engage in regular physical activity before trying to conceive again. In addition, early BG screening should occur with future pregnancies (ADA, 2023).
Secondary/Miscellaneous Diabetes
Secondary or miscellaneous diabetes is elevated BG levels caused by an illness, toxin, genetic abnormality, or medication. This type of diabetes occurs due to reduced insulin sensitivity or secretion and constitutes approximately 1-2% of all diagnosed cases of diabetes (Brutsaert, 2022b). While several disease processes and medications can cause secondary diabetes, some common examples are listed in Table 4.
Table 4
Common Diseases and Medications Associated with Secondary Diabetes
Disease Conditions | Medications |
Cushing’s syndrome | Corticosteroids |
Hyperthyroidism | Glucocorticoids |
Muscle disorders such as myotonic dystrophy | Niacin |
Liver disease | Estrogen |
Chronic pancreatitis or pancreatic cancer | Thyroid hormone |
Autoimmune disorders (i.e., systemic lupus erythematous) | Second-generation antipsychotics (i.e., clozapine [Clozaril] or olanzapine [Zyprexa]) |
Acromegaly | Phenytoin |
Cystic fibrosis | Calcineurin inhibitors |
Hemochromatosis | Beta-blockers |
(Brutsaert, 2022b; Sapra & Bhandari, 2023)
The signs and symptoms of secondary diabetes are consistent with those previously listed for T1DM and T2DM, although symptoms depend on the severity of the insulin insensitivity and hyperglycemia. If the cause is not apparent, this type of diabetes can be challenging to diagnose. These patients may present with DKA (similar to T1DM) or hyperglycemic hyperosmolar syndrome (HHS, similar to T2DM) or more minor symptoms, including polydipsia, polyphagia, or polyuria (Brutsaert, 2022b; Sapra & Bhandari, 2023).
Diagnostic Tests for Secondary Diabetes
Diagnostic tests for secondary diabetes may vary based on the etiology of the condition, although most patients will be diagnosed similarly to T1DM and T2DM. Starting with the random BG level, additional testing (i.e., antibody testing) may be needed to determine the optimal treatment plan for the patient with secondary diabetes. This diagnostic work-up and parameters mirror those discussed above for T1DM and T2DM. A thorough history and physical exam are indicated to determine any pharmacological or physical history that could indicate secondary diabetes (ADA, 2023; Brutsaert, 2022b; Sapra & Bhandari, 2023).
Treatment/Management of Secondary Diabetes
Immediate treatment to lower the BG level should be initiated with oral antidiabetic drugs or insulin. For secondary diabetes caused by medication, changing the medication regime should be considered if possible, eliminating the need for antidiabetic medications and other management. When due to a medical condition, treatment should focus on rectifying the underlying cause. For some patients with secondary diabetes, lifelong treatment will be needed. Diabetic education should be given, including nutrition, medication regimen, and lifestyle modifications needed for all newly diagnosed diabetic patients (Brutsaert, 2022b; Sapra & Bhandari, 2023).
Complications of DM
Prediabetes Complications
As previously stated, prediabetes carries an increased risk of CVD and other complications, and most patients are unaware of their diagnosis; thus, routine screening is crucial to identify those at risk and start early interventions that are both cost-effective and therapeutically beneficial. Dietary modifications and increased physical activity combined with blood pressure and cholesterol management can likely reverse the risk of developing T2DM, the associated complications, and the need for future costly interventions such as oral or subcutaneous hypoglycemic medications or insulins (ADA, 2023; USPSTF, 2021b).
GDM Complications
Individuals with GDM have an increased chance of developing T2DM at some point. Their risk can be lowered by maintaining a healthy weight, eating a healthy diet, and engaging in routine exercise. The individual with GDM is also at an increased risk for HTN, preeclampsia, hydramnios, and a cesarean delivery due to the potential for macrosomia or excessive birth weight. To decrease the risk of preeclampsia (HTN, peripheral swelling, and proteinuria during pregnancy), pregnant patients with T1DM or T2DM should be prescribed low-dose aspirin at a dose of 100 to 150 mg/day by 12 to 16 weeks gestation; due to the availability of 81 mg tablets a daily dose of 162 mg/day is acceptable. Patients with diabetes complicated by HTN (BP above 135/85 mmHg consistently) should be treated with a goal no lower than 110-135/80 mmHg; however, angiotensin-converting enzyme inhibitors (ACE inhibitors), angiotensin receptor blockers (ARBs), and spironolactone (Aldactone) should be avoided or stopped when pregnancy planning begins or at conception and changed to safer and more effective drugs for pregnancy including methyldopa (Aldomet), labetalol (Normodyne), or nifedipine (Procardia; ADA, 2023; Goyal & Jialal, 2023). Complications for the baby born to a mother with GDM include:
- macrosomia, over nine pounds at birth
- hypoglycemia at birth
- prematurity leading to respiratory issues
- congenital abnormalities
- childhood or adolescent obesity
- development of T2DM later in life (Goyal & Jialal, 2023)
For additional information regarding diabetes management in pregnant patients, please see the NursingCE course entitled Diabetes in Pregnancy.
T1DM/T2DM Complications
Hyperglycemia
Hyperglycemia occurs when an individual's BG is above 125 mg/dL while fasting and 180 mg/dL at two hours postprandial. Symptoms include polyuria, polydipsia, dry mouth, blurred vision, fatigue, and nausea. When hyperglycemia is left untreated for an extended period, damage to the eyes, kidneys, and cardiovascular system can result (Mouri & Badireddy, 2023). Hyperglycemia can occur for several reasons, including but not limited to:
- illness
- stress
- ingestion of high-GI or large quantities of food
- missed doses of medication
- decreased activity
- use of medications that increase BG (Mouri & Badireddy, 2023)
Patients with T1DM should have access to a home ketone testing kit using either urine or blood sample. Patients should be instructed to self-check for ketones if they experience symptoms of ketoacidosis (nausea or vomiting, abdominal pain, or flu-like symptoms) or if their BG is above 250 mg/dL. The APRN should instruct patients to call their healthcare provider if their BG level remains above 250 mg/dL for more than two consecutive checks (Brutsaert, 2022b).
Hypoglycemia
Hypoglycemia is a major consideration when attempting to achieve glycemic control. There are three levels of hypoglycemia. Level 1 hypoglycemia occurs when the BG is below 70 mg/dL to 54 mg/dL. Level 2 hypoglycemia is a BG under 54 mg/dL, and level 3 is hypoglycemia associated with altered mental or physical status requiring treatment. While it is more common with T1DM, hypoglycemia may occur with T2DM after taking insulin or certain medications that stimulate increased insulin production (i.e., sulfonylureas), engaging in more physical activity than normal, or skipping a meal (ADA, 2023). The individual suffering from hypoglycemia may exhibit the following signs and symptoms:
- sweating
- shakiness
- weakness
- hunger
- irritability
- dizziness
- headache
- blurred vision
- heart palpitations
- slurred speech
- drowsiness
- anxiety/nervousness
- confusion
- fatigue (ADA, 2023; Mathew & Thoppil, 2022)
The APRN should educate patients to recognize hypoglycemia quickly, and that management should include (ADA, 2023; Mathew & Thoppil, 2022):
- If conscious, the preferred treatment is a glucose tablet (15-20 g) if available. Alternatively, the patient should be instructed to eat or drink a simple sugar such as a serving of fruit juice, hard candy, or regular soda equivalent to 15-20 g of sugar.
- Retest BG in 15 minutes to ensure it is above 70 mg/dL. If not, repeat the step above. Avoid foods high in fat, as glucose absorption will be delayed in the presence of fat.
- Once the BG level is above 70 mg/dL or trending up, the individual should eat a meal or snack containing a complex carbohydrate to avoid another episode of hypoglycemia. In patients with T2DM, consuming protein can increase insulin response without a corresponding increase in plasma glucose levels. Due to this, sources of carbohydrates high in protein should be avoided when treating or preventing episodes of hypoglycemia.
- For the patient who loses consciousness during hypoglycemia (level 3) or those who are unable or unwilling to take oral treatment, caregivers should be instructed to administer glucagon intramuscularly or intranasally or place a simple sugar inside the gums. Once the individual regains consciousness, a complex carbohydrate food should be given.
APRNs must educate the patient and caregivers about the risk of this life-threatening condition. Hypoglycemia can be avoided through early recognition, intervention, and prevention. Patients on insulin with hypoglycemia unawareness, a single level-3 event, or a pattern of unexplained level-2 events should be instructed to increase their BG targets for at least several weeks to partially reverse the hypoglycemia unawareness and reduce future risk. Individuals in close contact with individuals at risk of hypoglycemia should be educated on the use of glucagon, including where it is kept and how to administer it. This includes roommates, school personnel, coworkers, childcare professionals, or correctional officers (ADA, 2023).
Diabetic Ketoacidosis
DKA is a hyperglycemic crisis that occurs when insulin levels are insufficient to meet metabolic requirements. This can lead to a diabetic coma or death. DKA is a complication that occurs primarily with T1DM but can occur in some patients with T2DM. DKA may be the first sign of T1DM in the patient unaware of their disease. Due to a lack of insulin to transport glucose into the cells, the body breaks down amino acids and triglycerides for energy, producing ketones. These ketones build up in the blood and make it more acidic. The rising ketones lead to DKA. Increased mortality and morbidity occur with DKA. Symptoms of DKA are discussed above in the section on T1DM. If symptoms of DKA are present, the patient should be instructed to seek healthcare immediately (Brutsaert, 2022c). Common causes of DKA include:
- infections such as pneumonia
- myocardial infarction
- stroke
- trauma
- missed dose of insulin
- pancreatitis
- pregnancy
- use of medications such as corticosteroids, thiazide diuretics, or sympathomimetics (Brutsaert, 2022c; El-Remessy, 2022)
Diagnostic criteria for DKA include a BG above 250 mg/dL, an arterial pH less than 7.3, a bicarbonate level less than 18 mEq/L, and an anion gap greater than 10. The presence of ketones in the serum or urine can also confirm a diagnosis of DKA. Emergency management of DKA is based on the restoration of circulating volume and the correction of BG levels. Fluid resuscitation is initiated with 2-3 L of 0.9% normal saline at 15-20 mL/kg/h, followed by 0.45% normal saline at 4-14 mL/kg/h. Once BG levels fall to 250 mg/dL or below, the intravenous solution should be changed to 0.45% normal saline with added dextrose (5% or 10%) to avoid hypoglycemia while ketoacidosis is corrected. Regular insulin is administered intravenously with an initial bolus of 0.1 unit/kg followed by a continuous infusion of 0.1 unit/kg/h. If BG does not decrease by 50 to 75 mg/dL after one hour, the insulin dose should be doubled. The continuous IV infusion of insulin should be adjusted based on BG levels. However, it should continue until the anion gap has narrowed on two consecutive blood tests and there are no ketones in the urine or blood. Once the patient is stable and can resume oral intake, a basal-bolus insulin regimen can be started with IV insulin discontinued 1 to 4 hours after the initial subcutaneous insulin administration. Potassium levels should be monitored throughout the acute phase of DKA to assess for hypokalemia, and replacement potassium given as appropriate. If the potassium level is less than 3.3 mEq/L, the insulin infusion should be held, and 20-30 mEq/h of potassium replacement should be administered until levels rise above 3.3 mEq/L. If potassium levels are 3.3-5.2 mEq/L, 20-30 mEq/h of potassium should be administered for each liter of fluid to maintain a potassium level of 4-5 mEq/L. If the potassium level is above 5.2 mEq/L, levels should be rechecked every 2 hours since insulin administration can cause rapid potassium shifts. Other electrolyte levels should be monitored throughout treatment and managed accordingly (Brutsaert, 2022c; El-Remessy, 2022).
Sick Days and Insulin
Diabetic patients experiencing a sick day should check their BG levels every three to four hours. T1DM patients should further check their urine for ketones if their BG is significantly elevated (above 250 mg/dL). Drinking about 8-12 ounces of water per hour consistently during sick days is vital to avoid dehydration. If patients have difficulty keeping fluids down, taking small sips every 15 minutes is advised. Patients should attempt to continue to drink, eat, and take their usual short-acting, intermediate, or premixed insulin dose; their bolus dose(s) should match their carbohydrate intake. Patients who cannot eat or drink should be instructed to take a correction factor dose every three to four hours. A correction factor dose decreases BG when it goes too high. Each patient should have an individualized correction dose determined by their healthcare provider and based on their current BG reading, but typically it is 50% of their traditional bolus dosing or 1 unit for each 50 mg/dL if their BG is less than 250 mg/dL. This should be adjusted based on various factors, including age, weight, or activity level. Patients that have moderate to high ketones present in their urine, cannot keep liquids down for over 4 hours or food down for over 24 hours, experience weight loss over 5 pounds, have a BG less than 60 mg/dL, have severe vomiting or diarrhea lasting longer than 6 hours, or a temperature above 101˚ F for over 24 hours should call their provider and seek medical attention at an emergency department (CDC, 2022h; Wood & Peters, 2018).
Hyperosmolar Hyperglycemic State
Hyperosmolar hyperglycemic state (HHS), formally known as hyperglycemic hyperosmolar nonketotic coma (HHNK), is a life-threatening condition primarily occurring in patients with T2DM when their BG level rises above 600 mg/dL. Some patients may present with HHS initially, previously unaware of their T2DM diagnosis. In diagnosed T2DM patients, this condition may be preceded by illness or infection and is typically accompanied by extreme dehydration and an altered level of consciousness. It may also be caused by missing doses of T2DM medications or taking a medication that decreases the effectiveness of insulin or diuretics. When checking BG levels, the glucometer may indicate "high." Affected individuals may present with a dry mouth, extreme thirst, confusion, weakness, nausea, weight loss, fever, hypotension, tachycardia, and seizures; if left untreated, the patient may become comatose. The mortality rate of HHS is 20%, compared to the mortality rate of DKA, which is less than 1%. Lab values typically indicate an absence of acidosis, as patients with T2DM are still making enough insulin to avoid ketogenesis. The primary difference between HHS and DKA is the lack of high ketone levels and marked metabolic acidosis. Despite this, the patient with HHS may have ketones in their blood or urine if the condition is severe. The dehydration associated with HHS can exceed 10L and is related to hyperglycemia-induced osmotic diuresis as the kidneys attempt to correct the elevated BG levels by increasing urine output. The APRN should instruct patients and caregivers to seek immediate care for any signs or symptoms of HHS. Emergent treatment for HHS mimics that of DKA, focusing on intravenous fluid replacement and insulin to reduce BG levels. Potassium replacement should be based on comprehensive metabolic profile results and institution-specific algorithms. Circulatory collapse, shock, cerebral edema, lactic acidosis, and blood clot formation are known complications to be aware of in patients being treated for HHS (Brutsaert, 2022e).
Cardiovascular Disease
CVD is the leading cause of morbidity and mortality in patients with diabetes. CVD due to diabetes has an estimated annual cost of $37.7 billion in the US. Patients with diabetes have double the risk of heart failure compared to non-diabetic patients and are affected earlier than non-diabetic patients. This is due to the effect of hyperglycemia on the vessels and nerves that feed and control the heart. HTN and hypercholesterolemia are both more common in patients with diabetes and should be diligently controlled to reduce the risk of CVD. Other ways to reduce the risk of CVD include a diet rich in fruits/vegetables, lean protein, whole grains, and lots of water. Diabetic patients should avoid processed foods, trans fats, sugary drinks, and alcohol. Patients that are overweight should attempt to lose 5% of their body weight to reduce their risk, but benefits may be obtained with as little as 3%, and additional benefits may be seen with a weight loss of 7%. Adult patients should exercise at a moderate intensity at least three days per week for at least 150 minutes total. If applicable, smoking cessation should be strongly encouraged (ADA, 2023; Brutsaert, 2022). For additional information regarding CVD in patients with diabetes, please see the NursingCE course, Cardiovascular Health and Prescribing for the Diabetic Patient.
Neuropathy
Diabetic neuropathy is defined as nerve damage related to the cumulative chronic effects of hyperglycemia and is the most common complication of diabetes. Age and the length of time that the patient has had diabetes are nonmodifiable risk factors for neuropathy, while glycemic control, being overweight, HTN, hypercholesterolemia, advanced kidney disease, alcohol use disorder, and smoking are all modifiable factors that also increase the risk. The nerves may become damaged due to hyperglycemia or secondary to reduced vascular supply. As many as one-half of all patients with diabetic peripheral neuropathy are asymptomatic. Symptoms typically develop gradually over years, although some may present suddenly (ADA, 2023; Brutsaert, 2022a; Quan, 2021).
Symptoms of peripheral neuropathy depend on the type and location of the nerves involved. Typical symptoms include pain, tingling, numbness, or weakness that begins in the feet, moves proximally up the legs, and may also affect the arms and hands. The pain may be described as pins and needles sticking into the feet or hands. There may be burning or shooting pain in the feet or hypersensitivity to touch, including with socks, shoes, gloves, or bed linens. Most patients describe symptoms that are bilateral and worse at night. Numbness and weakness may be part of the presentation of neuropathy as well. This may affect gait, balance, and muscle tone, increasing a patient's fall risk. The decreased sensation may lead to blisters/ulcers on the toes or feet that develop or worsen without the patient's awareness. Severe cases may lead to Charcot's foot, a deformity related to damaged tissues and bones in the feet. Examinations for patients with diabetes should include an annual assessment of peripheral sensation, including vibration, light touch, and temperature, as well as an assessment of lower extremity strength, gait, and balance. Additionally, a visual assessment of the patient’s feet is required, and many patients require regular care from a podiatrist. Vitamin B12 deficiency, which may occur secondary to metformin (Glucophage) use, may worsen symptoms of peripheral neuropathy and should be ruled out in patients on this treatment (APA, 2023; Brutsaert, 2022a).
Physical therapy should be encouraged to help prevent falls and improve gait. Topical anesthetics such as lidocaine (Lidoderm), capsaicin cream (Zostrix), and capsaicin transdermal patch (Qutenza) may be helpful to some with minimal adverse effects. Both can be prescribed in a patch for easy application. Antidepressants and anticonvulsant medications may be helpful in the symptomatic management of peripheral neuropathy, although there is no therapeutic cure for the condition. Pregabalin (Lyrica) and its precursor gabapentin (Neurontin) were initially designed to treat seizures but were found to reduce neuropathic pain. Certain tricyclic antidepressants (TCAs; i.e., nortriptyline [Pamelor], desipramine [Norpramin], imipramine [Tofranil], and amitriptyline [Elavil]) may be helpful in some patients by blocking the reuptake of norepinephrine and serotonin. They often cause drowsiness, increased appetite, weight gain, dry mouth, urinary retention, and constipation, and may cause orthostatic hypotension and cardiac arrhythmias (APA, 2023; Brutsaert, 2022a; Quan, 2021). These were included in the 2023 American Geriatric Society Beers Criteria update of potentially inappropriate medication in older adults due to their anticholinergic effects and should be avoided in patients over 65 if possible (2023 American Geriatrics Society Beers Criteria Update Expert Panel, 2023). Selective serotonin reuptake inhibitors (SSRIs) traditionally used for depression, such as citalopram (Celexa) and paroxetine (Paxil), and serotonin-norepinephrine reuptake inhibitors (SNRIs) duloxetine (Cymbalta) or venlafaxine (Effexor) may also be effective at reducing symptoms. These antidepressants may cause nausea, headache, dry mouth, insomnia, and sexual dysfunction and place the patient at risk for serotonin syndrome due to excessive serotonin levels. Serotonin syndrome may present with agitation, anxiety, confusion, high fever, sweating, fluctuating BP, and tachycardia and requires emergent medical attention as it can be lethal (APA, 2023; Brutsaert, 2022a; Quan, 2021).
Autonomic neuropathy affects the nerves that control internal organs, such as the heart, gastrointestinal tract, bladder, sexual organs, sweat glands, and eyes. Damage to these nerves can also lead to a poor sense of hypoglycemia, known as hypoglycemia unawareness. The classic early symptoms of hypoglycemia, such as confusion, dizziness, hunger, irritability, or nervousness, are not experienced. This may potentially lead to severe hypoglycemia with loss of consciousness. Other symptoms of autonomic neuropathy may vary depending on which nerves are damaged but include hypotension (especially orthostatic hypotension), tachycardia or variable heart rate, reduced sensation of angina during myocardial ischemia events, gastrointestinal symptoms (bloating, fullness, nausea, vomiting, constipation, diarrhea [including dumping syndrome], fecal incontinence, or dysphagia [difficulty swallowing]), a poor sensation of bladder fullness, urinary incontinence, sexual dysfunction (erectile dysfunction [ED], retrograde ejaculation, vaginal dryness), night sweats, gustatory sweating (increased sweating while eating), anhidrosis (reduced sweating), delayed pupillary response to light, or difficulty driving at night (APA, 2023; Brutsaert, 2022a; Quan, 2021).
If concerned, initial tests for autonomic neuropathy include assessing the patient's orthostatic blood pressure, which includes checking heart rate and BP after lying down or sitting for 5-10 minutes, immediately upon standing, and then again after several minutes of standing. A decrease of more than 20 mmHg in systolic or 10 mmHg in diastolic or the presence of symptoms (dizziness, loss of balance) indicates the presence of orthostatic hypotension. Patients with confirmed autonomic neuropathy affecting heart rate and BP should be encouraged to increase their fluid intake and potentially salt (sodium) intake if hypotensive. Increased physical exercise and compression stockings to improve blood flow may help improve symptoms; however, autonomic neuropathy increases the risk of exercise-induced injury. Therefore, cardiac function should be assessed before engaging in physical activity. To mitigate symptoms, individuals with autonomic neuropathy may find it helpful to raise the head of their bed while sleeping. They should be instructed to stand from a lying or seated position gradually and carefully, with support as needed from caregivers, furniture, or an assistive device (cane, walker) if indicated. The most critical management tool for autonomic neuropathy is enhanced glycemic control (ADA, 2023; Quan, 2021).
Patients with bladder or sexual symptoms indicative of autonomic neuropathy affecting the bladder or sexual function should be referred to a specialist, such as a urologist or a gynecologist. The urologist will assess the patient and likely perform urodynamic testing, including a cystometrogram (CMG) for bladder issues. Patients with urinary incontinence (UI) should be encouraged to avoid constipation, instructed in timed voiding and bladder training, and referred for pelvic floor PT if indicated. Numerous products ranging from pads to washable absorbent underwear and incontinence briefs may be helpful. Tablets can also be taken by mouth to reduce urine odor and enhance patients' comfort regarding incontinence odors. Skincare around the peroneal area in patients who are incontinent should include frequent cleansing, protective creams, and regular visual inspections for irritation/infection (NIDDK, 2021b).
Those with stress incontinence should be encouraged to lose weight if overweight or obese, as this may alleviate some symptoms. Frequency, urge incontinence, or urinary retention may be reported as well. Elevated BG levels and urinary retention contribute to more frequent UTIs. Some female patients with stress incontinence may benefit from a consultation for a pessary to be inserted into the vagina or may attempt to use disposable internal bladder supports (Impressa) that can be purchased over the counter and inserted into the vagina for up to 12 hours to support the urethra and prevent leaking. A longer-term solution for stress incontinence is injectable bulking agents injected near the urinary sphincter to prevent leaks. Stress incontinence in men can sometimes be treated with penile clamps/clips. Severe urinary retention may require intermittent self-catheterization (Agochukwu-Mmonu et al., 2020; Gordon et al., 2017; NIDDK, 2021b).
People with diabetes with UI should be conscious and aware of how much, what, and when they drink. Avoiding liquids right before bed will help with nocturia, and avoiding caffeine, alcohol, and carbonated beverages may also help (NIDDK, 2021b). Pharmacological options for urge incontinence/overactive bladder include (Lightner et al., 2019):
- Antimuscarinics (also called anticholinergics) may be helpful for those with urge incontinence, such as oxybutynin (Ditropan), solifenacin (Vesicare), and tolterodine (Detrol). However, they should be avoided in those with delayed gastric emptying or gastroparesis. These work by binding to muscarinic receptors in the detrusor, blocking acetylcholine and thus suppressing involuntary contractions of the bladder. They often cause traditional anticholinergic side effects, such as a dry mouth.
- b3-adrenergic agonists, such as mirabegron (Myrbetriq), work by relaxing the smooth muscles within the bladder wall and may be combined with solifenacin (Vesicare).
- Botulinum (Botox) is a neurotoxin that can be injected into the detrusor muscle, temporarily blocking nerve impulses and relaxing muscle contractions but often causing urinary retention as a side effect, requiring temporary, intermittent catheterization.
- Estrogen (Premarin) vaginal cream may be helpful in female patients with atrophic urethritis to reduce detrusor activity, but these are not typically a first-line treatment.
Individuals with persistent UI ineffectively treated with medications may be candidates for electrical nerve stimulation. Male patients with diabetes and an enlarged prostate may be prescribed an alpha-blocker or 5-alpha reductase inhibitor. Alpha-blockers (i.e., doxazosin [Cardura], prazosin [Minipress], terazosin [Hytrin]) work by blocking the effects of norepinephrine on alpha-1 adrenergic receptors and thus causing smooth muscle relaxation. Alpha-blockers may cause hypotension, dizziness, or headache due to reduced BP. 5-alpha reductase inhibitors (i.e., finasteride [Proscar], dutasteride [Avodart]) block the enzymatic conversion of testosterone into dihydrotestosterone. They may cause impotence, decreased libido, affect ejaculation, and gynecomastia. Surgical resection of the prostate may be required if lifestyle and medications are ineffective (Erdogan et al., 2022; NIDDK, 2021b). Surgical options for incontinence include:
- in patients with overflow incontinence, surgery may be recommended to unblock or restore the urethra (in men, this commonly involves a transurethral resection of the prostate or TURP)
- in individuals with stress incontinence, a bladder sling procedure may be performed to insert material (typically mesh, but may also be a donor or autologous tissue graft) between the vagina and urethra to provide additional support or a bladder neck suspension to attach the bladder neck to the ligament along the pubic bone
- in men with stress incontinence, an artificial urinary sphincter can be implanted. This is a fluid-filled cuff placed around the urethra and connected to a saline reservoir/balloon in the abdomen that is controlled by a pump inserted into the scrotum to allow for urination when needed
- a male version of the sling procedure is also available, during which synthetic mesh is placed under the urethra like a hammock and is most effective in patients with mild to moderate stress incontinence (NIDDK, 2021b)
Diabetic-related sexual dysfunction treatment should include a multidisciplinary approach, including urology, gynecology, endocrinology, and psychiatry. Men with diabetes are 3.5 times more likely to be affected by sexual dysfunction than those without. Of men with diabetes, 33% of those with T2DM and 20% of those with T1DM report erectile dysfunction. Men may also develop a condition known as Peyronie’s disease or penile curvature. This curvature is caused by scar tissue called "plaque" in the penis, making it curve when erect. Curves in the penis can cause pain and discomfort during intercourse. ED can co-exist with Peyronie's disease, making sex even more difficult for the patient. Low testosterone can also occur in men with diabetes, likely secondary to the decreased pituitary hormone levels responsible for stimulating testosterone production. This decrease in testosterone can cause or worsen other sexual problems. Low testosterone can cause ED and decreased libido (sex drive). Men with diabetes are often overweight and less active, which can further contribute to low testosterone levels. ED can be treated with medication, so APRNs need to inquire about sexual concerns with patients and encourage open communication. Testosterone therapy can improve the patient’s sex drive and energy levels but should be used judiciously due to the detrimental side effects of steroid usage. The main course of treatment for sexual disorders in patients with diabetes is phosphodiesterase type 5 inhibitors. The FDA has approved many medications for sexual dysfunction, particularly erectile dysfunction. This includes sildenafil (Viagra), avanafil (Stendra), tadalafil (Cialis), and vardenafil (Levitra, Staxyn). These medications block phosphodiesterase 5, the enzyme that breaks down cyclic guanosine monophosphate required for an erection. This action only occurs in the presence of nitrous oxide, which is released during sexual arousal. Therefore, phosphodiesterase inhibitors only work to achieve an erection in the presence of arousal. These medications come with significant adverse effects, such as headache, flushing, and dizziness, along with an FDA black box warning of the potential for sudden vision and hearing loss. These medications are also contraindicated with nitrates (Shindel & Lue, 2021; Van Cauwenberghe et al., 2022). The APRN should inquire about the use of herbal supplements and alternative medications when assessing the patient. For example, Yohimbe is a natural remedy used for ED that can cause a hypertensive crisis when taken with tyramine-containing foods (cured meats, red wine, caffeine, aged cheeses, overripe fruit, etc.). Other supplements (i.e., “horny goat weed”) should also be noted, as they are not regulated by the FDA and can contain ingredients that may be harmful to the patient (National Center for Complementary and Integrative Health, 2020). Other treatments for ED include intraurethral suppositories, intracavernosal injections, vacuum erectile devices (VED), or penile prosthesis surgery. A surgical penile prosthesis is generally reserved for patients not responsive to other therapies (Shindel & Lue, 2021).
One meta-analysis of 25 studies involving almost 4,000 women found that sexual problems may be related to low sexual desire (64%), vaginal dryness (70%), and painful sex related to decreased blood flow to the genitals and hormonal changes from diabetes (43%). Decreased blood flow can lead to reduced or no sensation in the genital area (36%), an inability to have an orgasm (50%), and an inability to become aroused or stay aroused. Vaginal dryness can lead to pain and discomfort with intercourse. Mental health implications of having diabetes can also be involved in the decreased sexual desire and response by female patients. Furthermore, some individuals may have recurrent vaginal yeast infections due to elevated BG and decreased immunity, leading to painful intercourse (Barbagallo et al., 2020; NIDDK, 2018a). Menopausal hormone therapy (MHT), such as estrogen replacement therapy, can benefit individuals with climacteric symptoms (hot flashes, insomnia, mood disturbances, vaginal dryness, and sexual dysfunction). In the past utilizing MHT has been controversial in patients with diabetes due to the view that T2DM has the same risk of adverse effects as CVD. However, new research indicates no link to increased mortality from CVD with MHT. Despite the lack of supporting evidence that MHT is harmful, many providers will not prescribe MHT to patients with T2DM. It is important to note that estrogen therapy has not been proven to increase sexual desire; however, it does help decrease vaginal dryness and dyspareunia. There is also strong evidence that MHT can lead to glucose homeostasis in patients with or without T2DM. Applications of this medication include vaginal creams, rings, and tablets. Patients who cannot partake in hormonal treatment due to a history of hormone-receptive cancers or who do not care for hormone therapy may be treated with water-based lubricants and moisturizers to help with dryness and discomfort (Paschou & Papanas, 2019; Thornton et al., 2015). Both sexual and bladder health can be improved by:
- maintaining therapeutic BG levels as determined by goals set with healthcare providers
- routine physical activity
- maintaining a healthy weight as determined by goals set with healthcare providers,
- quitting smoking
- seeking mental health interventions for any emotional or psychological symptoms (NIDDK, 2018a)
Significant others of the patient with diabetes should also be part of the conversation and active members of the healthcare team to offer the most support and understanding possible. Such personal concerns as sexual and bladder health can be embarrassing and cause anxiety in many patients, and a support system that involves their partner can help deal with the issues of concern (NIDDK, 2018a).
Delayed stomach emptying or gastroparesis can occur due to autonomic neuropathy that damages the vagus nerve. The vagus nerve controls the muscular activity of the stomach and intestines, leading to gastric motility. Slower movement of food through the digestive tract may lead to gastroesophageal reflux (GERD), heartburn, nausea, vomiting of undigested food, abdominal bloating, decreased appetite, erratic BG levels, abdominal pain, and early or prolonged satiety when eating. With gastroparesis, food stays in the stomach longer, delaying absorption; however, insulin absorption is not slowed, leading to increased difficulty maintaining euglycemia with potential severe hypoglycemia. Nutrients are not well absorbed, and patients with vomiting are at risk of dehydration and electrolyte disturbances. Further, food in the stomach for too long can lead to fermenting and bacterial overgrowth. The food can also harden into solid masses called bezoars, which can lead to nausea, vomiting, and obstruction, blocking the passage of food into the small intestines. Patients with gastroparesis should be instructed to avoid large amounts of indigestible cellulose found in celery, pumpkin, prunes, raisins, leeks, beets, persimmons, and sunflower-seed shells to reduce their risk of developing a bezoar (ADA, 2023; NIDDK, 2018b; Young et al., 2020).
Patients with diabetes should avoid medications that may further slow gastric emptying, such as narcotics, certain antidepressants (amitriptyline [Elavil], nortriptyline [Pamelor], and venlafaxine [Effexor]), anticholinergics, antimuscarinics (used to treat overactive bladder, such as oxybutynin [Ditropan]), and pramlintide (Symlin). The gold standard for gastroparesis diagnosis is gastric emptying scintigraphy. This involves consuming 120 grams of liquid egg whites, two slices of white toast, 30 grams of jelly, and 120 mL of water with 0.5 to 1.0 mCi of 99mTc-sulfur colloid, then undergoing testing every 15 minutes for 4 hours. Another test that can be used for diagnosis is the 13C octanoic acid breath test or gastric emptying breath test. Dietary modifications are the first-line treatment for diabetic gastroparesis. Patients diagnosed with diabetic gastroparesis should have a dietary consultation with a trained and experienced nutritionist to plan a diet that focuses on glycemic control, is low in fat and fiber, and is divided into four to five small meals throughout the day. Patients should be trained to take small bites, chew food thoroughly, eat softer foods that are well-cooked, and drink plenty of water and other non-carbonated fluids. Liquid meal replacements may be helpful. They should avoid carbonated beverages and alcohol. A walk or similar gentle physical activity after eating may aid gastric motility, and they should avoid lying down for at least two hours after eating. A dietary supplement such as a daily multivitamin may help restore nutritional deficiencies. Some patients with gastroparesis will switch to postprandial insulin injections instead of the traditional preprandial injection (APA, 2023; Aswath et al., 2023; Young et al., 2020). Certain medications may assist with increasing gastric motility:
- metoclopramide (Reglan) may increase gastric muscular contractions and reduce nausea and vomiting; it is the only FDA-approved medication for gastroparesis
- erythromycin (Erythrocin) is an antibiotic that may be used to increase muscle contractions within the GI tract
- ondansetron (Zofran), prochlorperazine (Compazine), promethazine (Phenergan), and OTC antiemetics such as bismuth subsalicylate (Pepto-Bismol) may be used to treat nausea and vomiting associated with gastroparesis, but do not increase gastric emptying
- mirtazapine (Remeron) is an antidepressant that may help alleviate nausea and vomiting but may not increase gastric emptying
- non-narcotic pain medications may be used to treat abdominal pain (narcotics should be avoided, as previously mentioned); examples include TCAs, tramadol (Ultram), or gabapentin (Neurontin; Aswath et al., 2023; Young et al., 2020)
In severe cases of gastroparesis, where malnutrition develops, tube feedings into the small intestine may be required. This can be done temporarily through a nasal or oral tube or a surgically placed jejunal or 'J' tube if longer-term feeding is required. Parenteral nutrition may be necessary if the jejunal tube is not effective. A venting gastrostomy is a surgical procedure in which a tube is placed into the stomach to relieve pressure and allow stomach contents to flow out when too full. A gastric electrical stimulator (GES) can be placed subcutaneously in the lower abdomen to electrically stimulate the muscles lining the stomach to decrease nausea and vomiting related to diabetic gastroparesis (Aswath et al., 2023; Young et al., 2020).
Focal neuropathies (or mononeuropathies) are less common than peripheral or autonomic neuropathies; they affect a single nerve, most often in the hand, wrist, head, torso, or leg. Entrapments are the most common type of focal neuropathy in which a nerve becomes compressed when passing through a small space between bones and other tissues. Diabetic patients are at increased risk for nerve entrapment. One example is carpal tunnel syndrome, in which the median nerve is entrapped or compressed by the transverse carpal ligament in the wrist, causing progressively worsening tingling, numbness, or pain in the thumb, index, and middle fingers (Brutsaert, 2022a; Zaino et al., 2023). Other focal neuropathies include:
- ulnar nerve palsy (cubital tunnel syndrome) is ulnar entrapment at the elbow, causing symptoms that radiate down into the pinky and ring fingers
- entrapment of the common peroneal nerve (peroneal nerve palsy) causing neuropathy symptoms into the lateral lower leg and the space between the 1st and 2nd metatarsals
- cranial neuropathies (causing various symptoms depending on the affected nerve, such as unilateral eye pain, double vision/difficulty focusing the eyes, or unilateral facial paralysis [Bell’s palsy]; Rubin, 2022)
Nerve conduction studies or electromyograms (EMGs) may be done to confirm a focal neuropathy if the clinical diagnosis is inconclusive and assess severity. Various splints or braces may help maintain a neutral position across a joint (such as the wrist or elbow) to allow the nerve time to heal and inflammation to subside. Anti-inflammatory medications such as corticosteroids and nonsteroidal anti-inflammatory drugs (NSAIDs) may help relieve the symptoms temporarily. However, surgical decompression may become necessary if chronic irritation and inflammation persist or progress due to entrapment despite more conservative treatments (Rubin, 2022).
Retinopathy and Other Eye Complications
Diabetic retinopathy is a complication that affects individuals with T1DM and T2DM. It is the most common cause of blindness in individuals between 20 and 74. The degree of retinopathy and age of onset depends on BG control, duration of diabetes, lipid levels, and BP control. Due to the vascular changes in the eye, individuals with diabetes have an increased risk of glaucoma, cataracts, and other eye conditions with an age of onset younger than those without diabetes. Retinopathy may be nonproliferative or proliferative. Nonproliferative retinopathy is the most common and develops first. Capillary blood flow to the back of the eye is blocked, which leads to the capillaries swelling and forming microaneurysms which may leak fluid into the retina. This fluid leakage can lead to macular edema or thickening of the retina. As more blood vessels are damaged, nonproliferative retinopathy may progress to proliferative retinopathy over the years. As more blood vessels are blocked, new blood vessels start growing in the retina, but these tend to be weak and leak blood, leading to vitreous hemorrhage. The progressive damage can also cause scar tissue to form, pulling the retina out of place and causing traction retinal detachment (Mehta, 2022). Figure 3 demonstrates the changes that occur in the eye due to diabetic retinopathy.
Figure 3
Diabetic Retinopathy
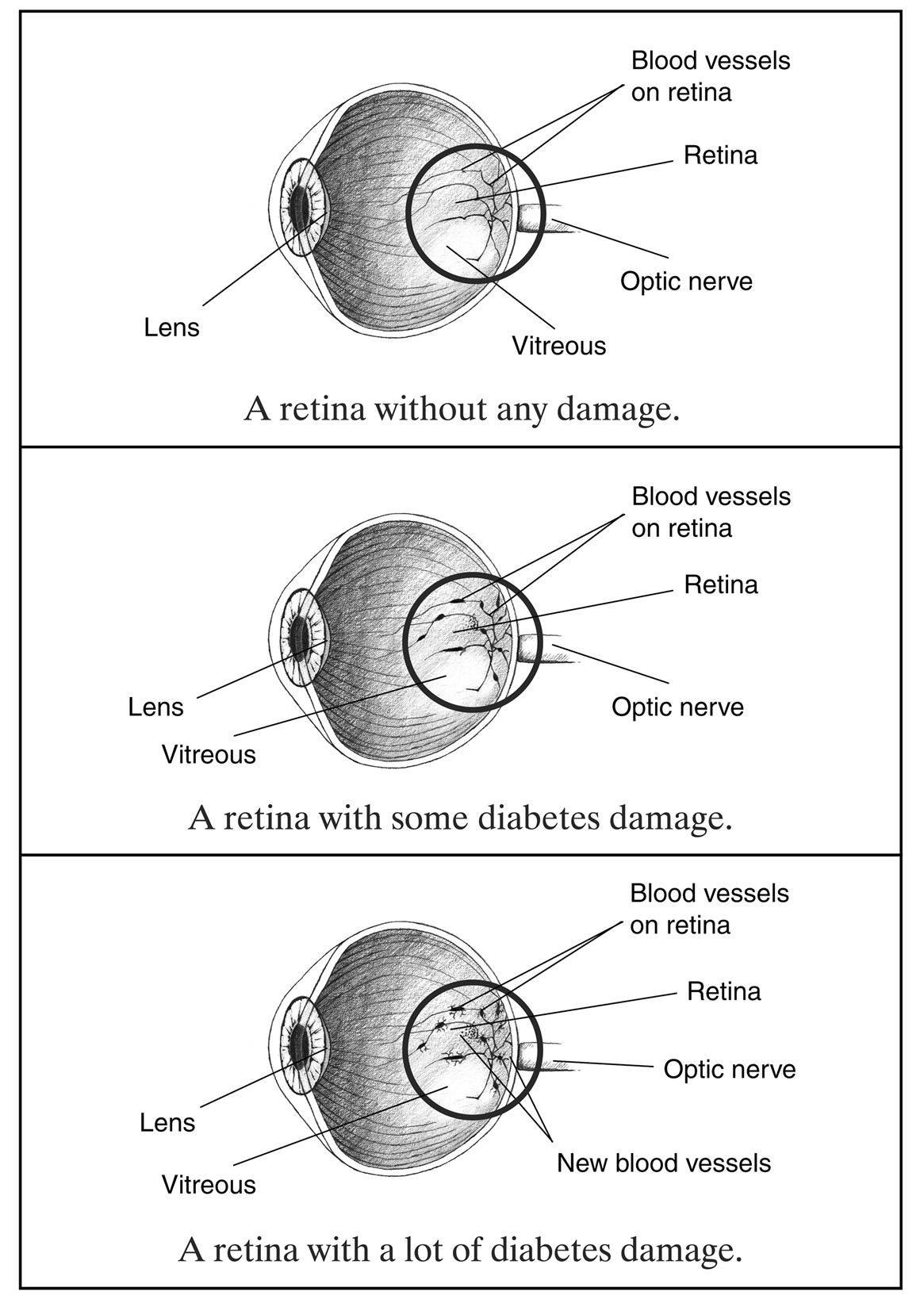
(NIDDK, n.d.-b)
Panretinal laser photocoagulation therapy can decrease the likelihood of vision loss in both retinopathies. Treatment with anti-vascular endothelial growth factor agents injected into the eye has been shown to regress proliferative retinopathy. Currently, only aflibercept (Eylea, Zaltrap) and ranibizumab (Byooviz) are FDA approved for this purpose. Surgical intervention with a vitrectomy may be considered in patients with retinal detachment, persistent vitreous hemorrhage, or extensive preretinal membrane formation. This procedure removes the vitreous humor gel that fills the eye cavity to gain better access to the retina. This allows the removal of scar tissue, laser repair of retinal detachments, and treatment of macular holes. Despite the availability of medical and surgical procedures to correct retinopathy, prevention via vigilant BG and BP control should be the first line of treatment (ADA, 2023; Mehta, 2022).
Nephropathy
Renal complications are one of the costliest aspects of long-term diabetes. In 2019, the cost of treating chronic kidney disease (CKD) by Medicare beneficiaries alone was estimated at $87.2 billion, with ESRD costing $37.3 billion (CDC, 2022a). The kidneys are very vascular, with tiny vessels that filter out the waste products from the blood. Due to microvascular damage, the kidneys may gradually lose the ability to filter out waste, eventually necessitating dialysis. Approximately 20%-40% of diabetic patients develop CKD. Due to the prevalence of CKD with diabetes, individuals diagnosed with diabetes should have their urinary albumin level assessed at the time of diagnosis and then annually. Factors contributing to kidney disease include HTN, poor glycemic control, and genetics. Patients can decrease their risk of kidney damage by maintaining therapeutic BG levels and controlling HTN. Patients with diabetes are also at increased risk for UTIs and should be instructed to report any symptoms immediately. Unfortunately, kidney disease is often asymptomatic until advanced stages, so urinalysis to check for proteinuria in diabetic patients is crucial to screening for kidney damage. Diabetic kidney disease also increases the risk of hyperkalemia, so electrolyte levels should be monitored closely. Kidney disease may present with fluid retention (pitting edema, sudden weight gain), insomnia, poor appetite, nausea, weakness, and difficulty concentrating. Control of BP and BG can also slow the progression of kidney disease. Patients who stay well-hydrated and eat a low-protein and low-sodium diet may be able to reduce both their BP and the workload of their kidneys, especially patients with macroalbuminuria (elevated protein in the urine). Unfortunately, once ESRD occurs, the only definitive treatment options include dialysis and renal transplant (ADA, 2023; Brutsaert, 2022a). For additional information regarding kidney disease, please see the NursingCE course entitled Chronic Kidney Disease.
Hypertension and Stroke
CVD is a complication frequently associated with diabetes. HTN is a significant risk factor for developing CVD and is commonly associated with a diagnosis of diabetes. HTN increases the risk of stroke, cardiac disease, and kidney disease. By lowering the BP, the risk of these complications declines or is delayed. The ADA identifies the ideal BP as 130/80 or less. Blood pressure should be monitored at each follow-up visit to implement early intervention (APA, 2023). The APRN should counsel patients regarding the following tips to reduce their BP:
- eat more whole-grain bread and cereals
- eat a low-sodium diet
- maintain a healthy weight
- limit or avoid alcohol intake
- engage in physical activity (ADA, 2023)
APRNs should choose antidiabetic medications carefully, especially in patients with comorbid HTN. As previously mentioned, SGLT2 inhibitors and, to a lesser extent, GLP-1 receptor agonists may cause a reduction in BP in T2DM patients. If medications are required to reduce BP (130/80 and above per the AHA and the ADA), ACE inhibitors, ARBs, diuretics, and calcium channel blockers (CCBs) have been shown to reduce BP and slow kidney disease without elevating BG in patients with diabetes. Hyperkalemia is a concern for patients with diabetes and kidney disease, especially for those taking an ACE inhibitor or ARB, and this should be monitored closely. Despite this, the current practice guidelines recommend an ACE inhibitor or an ARB for patients with diabetes and albuminuria. Thiazide-like diuretics such as HCTZ (Hydrodiuril) can be effective in reducing volume as well as reducing systemic potassium levels. With advanced renal disease and an eGFR of 30 mL/min/1.73 m2 or less, a long-acting loop diuretic is preferred, such as torsemide (Demadex). The ADA and AHA recommend treating HTN to a goal of less than 130/90 in patients with diabetes to reduce the risk of cardiovascular complications (ADA, 2023; Whelton et al., 2017). For additional information regarding hypertension, please see the NursingCE course entitled Hypertension: Diagnosis and Management.
Patients with diabetes have a 1.5-2 times greater risk of stroke when compared to individuals without diabetes. Further modifiable risk factors for stroke include being overweight, hyperlipidemia including high LDL or low HDL cholesterol, a sedentary lifestyle, tobacco use, and alcohol use. The APRN should encourage diabetic patients to lower their stroke risk with regular physical activity, smoking cessation, maintaining a healthy weight, and managing any existing hypercholesterolemia and HTN. One meta-analysis of 73,913 patients with diabetes demonstrated a reduction of stroke by 39% in individuals able to maintain a systolic BP of less than 130 mmHg. Like other diabetic complications, the patient’s overall risk can be lowered by maintaining therapeutic BG levels (Mosenzon et al., 2023; Whelton et al., 2017). For additional information regarding stroke, please see the NursingCE course entitled Stroke.
Skin Complications
Diabetes impacts every part of a patient's body, including their skin. Most skin conditions can be prevented or easily managed with proper treatment. Good skincare and BG control can decrease this risk. Skin conditions in diabetic patients include diabetic dermopathy (DD), which presents with brown, scaly, or shiny round or oval-shaped lesions found on the anterior distal surface of the lower legs that are usually painless and do not require specific treatment. DD is one of the most common dermatologic complications associated with diabetes affecting approximately 50% of patients. Necrobiosis lipoidica diabeticorum (NLD) presents similar to dermopathy, but the lesions are more significant, and there are typically fewer of them. They initially present as dull, red, and raised but then progress to shiny lesions with a violet border that may become itchy, painful, or open. NLD is rare, affecting only 1% of patients with diabetes. NLD is chronic and can lead to disfigurement. The condition is challenging to manage, and treatment is only required to prevent or treat a secondary infection if the lesions open. Bullosis diabeticorum (BD), or diabetic blisters, are rare and typically present on the fingers, hands, toes, and feet (occasionally on the forearm or lower legs). They are painless with no surrounding redness and are most often seen in diabetic patients with neuropathy. They typically heal within a few weeks without any specific treatment, but BG control should be improved to facilitate healing and prevent future blisters from developing. Eruptive xanthomatosis is associated with hypercholesterolemia and presents with itchy, firm yellow bumps on the skin surrounded by a red halo. It is often seen on the hands, feet, arms, legs, or buttocks in young male patients with T1DM. As with diabetic blisters, lesions will typically resolve with improved glycemic control. Digital sclerosis occurs in about one-third of patients with T1DM. It is characterized by tight, thick skin that may reduce mobility and flexibility on the back of the hands, fingers, toes, and forehead. The only treatment for sclerosis is improved glycemic control. Generalized or disseminated granuloma annulare may present with red-brown or skin-colored patches of small papules arranged in rings or arcs. Granuloma patches may respond to antimalarials, retinoids, corticosteroids, cyclosporine, or calcineurin inhibitors; however, most treatment recommendations are derived from studies with small sample sizes; therefore, improved glycemic control is preferred. While patients with diabetes can experience these conditions, most are rare. Most skin problems experienced by patients with diabetes include bacterial or fungal infections or itching (ADA, n.d.-a; Labib et al., 2022).
Bacterial infections are typically caused by Staphylococcus bacteria, which may cause styes, boils, folliculitis, carbuncles, or nail infections. These infections may present with red, warm, swollen, and tender skin and typically require topical or systemic antibiotics. Candida albicans most often cause fungal infections in diabetic patients. Common varieties include jock itch (groin region), ringworm, athlete’s foot, or vaginal infections. Fungal infections are often very red, moist, and itchy and may include the presence of tiny blisters or scales. They often occur in moist skin folds, such as under the breasts, between fingers and toes, around the nails, in the corners of the mouth, in the armpits, and in the groin. Mild infections can typically be treated with a topical antifungal ointment (ADA, n.d.-a; Labib et al., 2022).
Itching is common in patients with diabetes due to dry, cracked skin and poor circulation (typically seen bilaterally in the lower legs). Areas commonly affected include the scalp, feet or ankles, trunk, and genitalia. The presence of itching may also be related to a fungal infection. Most itching can be controlled by instructing patients to limit the frequency of bathing, use a mild soap, and always apply a moisturizer to their skin after bathing. When applying a moisturizing lotion, the APRN should be careful to educate the patient to avoid applying lotion between the toes, as this can increase the moisture level and the patient's risk for infection. Possible treatments include the use of topical capsaicin or ketamine-amitriptyline-lidocaine compounded ointments or oral anticonvulsants such as gabapentin (Neurontin) or pregabalin (Lyrica; ADA, n.d.-a; Labib et al., 2022).
Acanthosis nigricans presents as hyperpigmented, velvety plaques seen most often in patients with T2DM that are overweight. They appear symmetrically, most often on the back of the neck, axilla, or groin, but may also be seen on the hands (known as tripe palms), elbows, and knees. While topical treatments such as retinoids (0.1% tretinoin [Retin-A] or adapalene [Differin]), vitamin D analogs (Calcipotriene), keratolytics (ammonium lactate), and chemical peels work for some, results can be variable. Oral retinoids such as isotretinoin (Accutane) and acitretin (Soriatane) may be effective, as well as certain oral antidiabetic medications such as metformin (Glucophage) and thiazolidinediones. Addressing the underlying condition with weight loss and improved glycemic control is the most effective treatment option (Labib et al., 2022).
Wound Care. Diabetic patients are at higher risk of developing wounds or other skin conditions that lead to wounds, and their wound healing time is prolonged due to the complications of diabetes. Elevated BG levels delay healing, and bacteria thrive in the high-glucose environment. Poor circulation further impedes healing in diabetic patients, leading to the risk of amputation for even minor wounds in the lower extremities. Maintaining euglycemia is vital to wound healing in diabetic patients. More aggressive wound treatments, such as negative pressure wound therapy, hyperbaric oxygen therapy, silver cream, extracorporeal shockwave therapy, or higher-cost dressing treatments such as bioengineered allogeneic cellular therapies, may be needed to ensure healing and avoid further complications (ADA, 2023). For more on wound care, refer to the NursingCE course entitled Interdisciplinary Wound Care.
Foot Care. The APRN should educate patients on the implications of elevated BG levels on their feet and legs. Skin changes can cause the feet to become dry and cracked. Diabetic neuropathy may contribute to reduced sensation in the feet. Blisters, sores, or cuts may not be felt, or burning hot water may go unnoticed. Due to poor circulation, ulcers may develop, and the healing process may be delayed, eventually leading to an infection that could put the patient at risk of amputation (ADA, n.d.-a, 2023). Patients should be instructed to do the following daily to avoid this outcome (CDC, 2023a):
- Inspect the feet daily for cuts, cracks, redness, edema, blisters, calluses, splinters, or dark spots. The primary healthcare provider should be notified if foot lesions do not heal within one day.
- Discuss care of corns and calluses with a podiatrist or primary healthcare provider if needed.
- Wash feet daily in warm water and dry thoroughly. Avoid hot water as burns can occur more quickly due to neuropathy and reduced temperature sensation.
- Toenails should be filed or cut straight across and filed to avoid sharp edges. Soak feet first to soften toenails. Avoid cutting toenails too short or rounded, which may lead to ingrown toenails.
- Avoid lotion between the toes, which can lead to skin breakdown or fungal infections. However, the heels and top of the feet should be moisturized to prevent cracking or dry skin, which can cause breakdown.
- Cotton socks should be worn daily to avoid blisters or sores from shoes. Socks should be clean and dry without seams, if possible.
- Good-fitting shoes should be worn to avoid friction and pressure sores. New shoes should be broken in slowly by wearing them for one to two hours daily for two weeks.
- Do not go barefoot in or out of the house. Slippers or shoes should be worn to avoid injury to the feet.
- Protect feet from extreme heat or cold.
- Elevate the feet when sitting to promote blood flow. Avoid crossing your legs while sitting. Wiggle toes and move ankles up and down for five minutes three to four times daily to increase circulation.
- Stop smoking, as this causes damage to the circulatory system, leading to poor circulation to the feet and legs.
- Manage BG, BP, and cholesterol levels to promote circulation and decrease vascular damage.
- Engage in routine exercise to promote circulation to the lower extremities and decrease damage from diabetes.
- Schedule an annual podiatrist evaluation.
Oral Health
Patients with diabetes experience oral health conditions such as xerostomia (dry mouth), oral candidiasis, and burning mouth syndrome. Increased BG also leads to increased sugar in the saliva, increasing the risk for dental cavities and gum disease, which can lead to tooth loss. Diabetic patients should be encouraged to brush their teeth at least twice daily, floss at least once daily, and get dental cleanings with a check-up at least yearly but preferably every six months. Patients with diabetes who have dentures should remove and clean them at least daily (CDC, 2022g).
Mental Health and Diabetes
Diabetes is a lifelong chronic illness that requires daily monitoring, treatment, acceptance, and lifestyle changes. Mental health issues can lead to depression and non-compliance with treatment. Untreated mental health conditions can make lifestyle modifications more difficult, or a diagnosis of diabetes can exacerbate mental health disorders such as depression, anxiety, or eating disorders. Due to the impact that a diagnosis of diabetes has on an individual, psychosocial screening should be incorporated into provider visits with a referral to a qualified healthcare professional when indicated (APA, 2023). The APRN needs to educate the patient regarding signs or symptoms of depression or stress, such as:
- sadness
- anhedonia (loss of interest in activities of interest previously enjoyed)
- over or under-eating
- insomnia or sleeping continuously
- difficulty making decisions
- extreme fatigue
- hopelessness, irritability, anxiety, or guilt
- aches, pains, or digestive problems not associated with other conditions
- having thoughts of suicide or death (Habib et al., 2022)
Diabetes distress is prevalent in patients with diabetes. It has some overlapping symptoms with depression but is specific to the emotional and mental burden of managing diabetes daily, including worry, frustration, and discouragement. Traditional antidepressant medications do not seem to improve the symptoms of diabetes distress. If left untreated, diabetes distress may lead to increased A1C levels, decreased adherence to medication management, and changes in lifestyle, such as decreased activity and poor eating habits (ADA, 2023). Interventions that have been linked to improvement include:
- treatment with a mental health counselor familiar with patients being treated for chronic health conditions and their unique needs
- session(s) with a diabetic educator to strategize and problem-solve concerns
- a focus on small steps, such as one to two specific diabetic management goals
- participation in a local or virtual support group for diabetes patients
- regular consultations with an endocrinologist (CDC, 2023b)
Emergency Preparedness for Diabetics
Individuals with diabetes should always be prepared with sufficient medication and supplies to manage their condition correctly. During natural disasters, emergencies, a pandemic requiring lockdown, or other hazards, the individual with diabetes must be prepared with enough supplies and medications to last at least a week. The CDC and ADA have extensive Emergency Preparedness resources with specific patient advice. After the devastating effects of Hurricane Katrina, the American Association of Clinical Endocrinology (AACE) created the My Diabetes Emergency Plan, a checklist of essential items a person with diabetes should have available (AACE, n.d.). The following is included in the checklist:
- a detailed list of all medical conditions, allergies, medications, most recent laboratory results, and contact information for their preferred pharmacy, healthcare providers, and at least two emergency contacts
- at least one week’s supply of all medications (a 30-day supply is preferred)
- at least one week’s supply of test strips, alcohol swabs, two glucometers, and extra batteries
- a cooler and re-freezable gel packs to store insulin
- empty plastic bottles or sharps containers to dispose of used needles, syringes, and lancets
- treatment for hypoglycemic episodes, including carbohydrates, glucose tablets, juice boxes, soda, hard candy, or glucose gel
- at least a two-day supply of nonperishable food and a three-day water supply (AACE, n.d.)
Individuals with diabetes should also know their rights related to the Americans with Disabilities Act and Section 504 of the Rehabilitation Act, which requires shelters to accommodate them with a service dog, use of sharps, or anything related to diabetes care. If possible, individuals with diabetes should find a special medical needs shelter that is more equipped to address their specific needs (CDC, 2022b)
Future Opportunities for the Diabetic
An artificial pancreas that would replace the body’s pancreatic function is being researched. This fully automated treatment measures BG levels and injects the right amount of insulin, mimicking a healthy pancreas. This option can replace the human component of measuring BG and has been shown to maintain steadier glycemic control, especially overnight. Further research with this treatment option explores different artificial pancreas systems, including a dual hormone delivery device that contains and releases insulin and glucagon and different insulins that can effectively change BG levels quickly (NIDDK, 2021a).
Researchers are also making progress in using stem cells to treat T1DM. Researchers at the Harvard Stem Cell Institute (HSCI) developed VX-880, an islet cell replacement therapy originating from stem cells. VX-880 demonstrated significant restoration of islet cell function by day 90 after treatment initiation when used with immunosuppressive drugs. After one infusion of half the optimal dose of VX-880, the subject of the trial, an individual with a 40-year history of T1DM, demonstrated increased fasting c-peptide levels, tighter glycemic control demonstrated by decreased A1c, and reduced insulin requirements (HSCI, 2021).
References
2023 American Geriatrics Society Beers Criteria. (2023). American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. Journal of the American Geriatrics Society, 1-30. https://doi.org/10.1111/jgs.18372
Agochukwu-Mmonu, N., Pop-Busui, R., Wessells, H., & Sarma, A. V. (2020). Autonomic neuropathy and urologic complications in diabetes. Autonomic Neuroscience, 229(1027736). https://doi.org/10.1016/j.autneu.2020.102736
American Association of Clinical Endocrinology. (n.d.). Are you prepared to manage your diabetes in an emergency? Retrieved July 6, 2023, from https://www.aace.com/disease-and-conditions/diabetes/are-you-prepared-manage-your-diabetes-emergency
American Diabetes Association. (n.d.-a). Diabetes and skin complications. Retrieved June 25, 2020, from https://diabetes.org/diabetes/skin-complications
American Diabetes Association. (n.d.-b). eAG/A1C conversion calculator. Retrieved June 26, 2023, from https://professional.diabetes.org/diapro/glucose_calc
American Diabetes Association. (n.d.-c). Insulin pumps: Relief and choice. Retrieved June 28, 2023, from https://diabetes.org/healthy-living/medication-treatments/insulin-other-injectables/insulin-pumps-relief-and-choice
American Diabetes Association. (n.d.-d). Learn the genetics of diabetes. Retrieved June 16, 2023, from https://www.diabetes.org/diabetes/genetics-diabetes
American Diabetes Association. (2018). Economic costs of diabetes in the US in 2017. Diabetes Care, 41(5), 917–928. https://doi.org/10.2337/dci18-0007
American Diabetes Association. (2023). Standards of care in diabetes—2023. Diabetes Care, 46 (Supplement 1). https://diabetesjournals.org/care/issue/46/Supplement_1
Association of Diabetes Care and Education Specialists. (n.d.). Find an accredited diabetes self-management education and support program in your area. Retrieved June 16, 2023, from https://nf01.diabeteseducator.org/eweb/DynamicPage.aspx?WebCode=DEAPFindApprovedProgram
Aswath, G. S., Foris, L. A., Ashwath, A. K., & Patel, K. (2023). Diabetic gastroparesis. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK430794
Bahar, S. G., & Devulapally, P. (2023). Pancreas transplantation. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK562338
Bellin, M. D., & Dunn, T. B. (2020). Transplant strategies for type 1 diabetes: Whole pancreas, islet and porcine beta cell therapies. Diabetologia, 63, 2049-2056. https://doi.org/10.1007/s00125-020-05184-7
Benson, G., & Hayes, J. (2020). An update on the Mediterranean, vegetarian, and DASH eating patterns in people with type 2 diabetes. Diabetes Spectrum, 33(2), 125-132. https://doi.org/10.2337/ds19-0073
Brutsaert, E. F. (2022a). Complications of diabetes mellitus. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/complications-of-diabetes-mellitus
Brutsaert, E. F. (2022b). Diabetes mellitus (DM). Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/diabetes-mellitus
Brutsaert, E. F. (2022c). Diabetic ketoacidosis (DKA). Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/diabetic-ketoacidosis-dka
Brutsaert, E. F. (2022d). Drug treatment of diabetes mellitus. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/drug-treatment-of-diabetes-mellitus
Brutsaert, E. F. (2022e). Hyperosmolar hyperglycemic state (HHS). Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/hyperosmolar-hyperglycemic-state-hhs
Centers for Disease Control and Prevention. (2022a). Chronic kidney disease basics. https://www.cdc.gov/kidneydisease/basics.html
Centers for Disease Control and Prevention. (2022b). Diabetes care during emergencies. https://www.cdc.gov/diabetes/library/features/diabetes-care-during-emergencies.html
Centers for Disease Control and Prevention. (2022c). Diabetic risk factors. https://www.cdc.gov/diabetes/basics/risk-factors.html
Centers for Disease Control and Prevention. (2022d). Diabetes self-management education and support (DSMES) toolkit. https://www.cdc.gov/diabetes/dsmes-toolkit/index.html
Centers for Disease Control and Prevention. (2022e). DSMES for health care providers. https://www.cdc.gov/diabetes/dsmes/dsmes-health-pro.html
Centers for Disease Control and Prevention. (2022f). Health and economic benefits of diabetes interventions. https://www.cdc.gov/chronicdisease/programs-impact/pop/diabetes.htm
Centers for Disease Control and Prevention. (2022g). How to promote oral health for people with diabetes: 5 actions for health care teams. https://www.cdc.gov/diabetes/professional-info/health-care-pro/diabetes-oral-health.html
Centers for Disease Control and Prevention. (2022h). Managing sick days. https://www.cdc.gov/diabetes/managing/flu-sick-days.html
Center for Disease Control and Prevention. (2022i). National and state diabetes trends. https://www.cdc.gov/diabetes/library/reports/reportcard/national-state-diabetes-trends.html
Centers for Disease Control and Prevention. (2022j). National diabetes prevention program: Information for healthcare professionals. https://www.cdc.gov/diabetes/prevention/info-hcp.html
Centers for Disease Control and Prevention. (2022k). National diabetes statistics report: Estimates of diabetes and its burden in the United States. https://www.cdc.gov/diabetes/data/statistics-report/index.html
Centers for Disease Control and Prevention. (2023a). Foot health. https://www.cdc.gov/diabetes/library/features/healthy-feet.html
Centers for Disease Control and Prevention. (2023b). Diabetes and mental health. https://www.cdc.gov/diabetes/managing/mental-health.html
Centers for Disease Control and Prevention Diabetes Prevention Recognition Program. (2021). Standards and operating procedures. https://www.cdc.gov/diabetes/prevention/pdf/dprp-standards.pdf
Colberg, S. R., Sigal, R. J., Yardley, J. E., Riddell, M. C., Dunstan, D. W., Dempsey, P. C., Horton, E. S., Castorino, K., & Tate, D. F. (2016). Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care, 39(11), 2065-2079. https://doi.org/10.2337/dc16-1728
Corcoran, C., & Jacobs, T. F. (2022). Metformin. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK518983
Divers, J., Mayer-Davis, E. J., Lawrence, J. M., Isom, S., Dabelea, D., Dolan, L., Imperatore, G., Marcovina, S., Pettitt, D. J., Pihoker, C., Hamman, R. F., Saydah, S., & Wagenknecht, L. E. (2020). Trends in incidence of Type 1 and Type 2 diabetes among youths - selected counties and Indian reservations, United States, 2022-2015. Morbidity and Mortality Weekly Report, 69(6), 161-165. http://dx.doi.org/10.15585/mmwr.mm6906a3
El-Remessy, A. B. (2022). Diabetic ketoacidosis management: Updates and challenges for specific patient population. Endocrines, 3(4), 801-912. https://doi.org/10.3390/endocrines3040066
Erdogan, B. R., Liu, G., Arioglu-Inan, E., & Michel, M. C. (2022). Established and emerging treatments for diabetes-associated lower urinary tract dysfunction. Naunyn-Schmiedeberg's Archives of Pharmacology, 395, 887-906. https://doi.org/10.1007/s00210-022-02249-9
Evert, A. B., Dennison, M., Gardner, C. D., Garvey, W. T., Lau, K. H. K., MacLeod, J., Mitri, J., Pereira R. F., Rawlings, K., Robinson, S., Saslow, L., Uelmen, S., Urbanski, P. B., & Yancy, W. S., Jr. (2019). Nutrition therapy for adults with diabetes or prediabetes: A consensus report. Diabetes Care, 42(5), 731-754. https://doi.org/10.2337/dci19-0014
Frommer, L., & Kahaly, G. J. (2020). Type 1 diabetes and associated autoimmune disease. World Journal of Diabetes, 11(11), 527-539. https://doi.org/10.4239/wjd.v11.i11.527
Gallagher, E., & Siu, H. Y. (2020). Diabetic ketoacidosis as first presentation of type 1 diabetes mellitus in a young child. Canadian Family Physician, 66(6), 425-426. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7292523
Gordon, B., Shorter, B., Isoldi, K. K., & Moldwin, R. M. (2017). Obesity with comorbid stress urinary incontinence in women: A narrative review to inform dietetics practice. Journal of the Academy of Nutrition and Dietetics, 117(6), 889-907. https://doi.org/10.1016/j.jand.2016.09.024
Goyal, R., & Jialal, I. (2023). Type 2 diabetes. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK513253
Habib, S., Sangaraju, S. L., Yepez, D., Grandes, X. A., & Manjunatha, R. T. (2022). The nexus between diabetes and depression: A narrative review. Cureus, 14(6), e25611. https://doi.org/10.7759/cureus.25611
Harding, M. M. (2020). Lewis's Medical-surgical nursing: Assessment and management of clinical problems (11th ed.). Elsevier.
Harvard Stem Cell Institute. (2021). A new therapy for treating type 1 diabetes. https://hsci.harvard.edu/news/new-therapy-treating-type-1-diabetes
Janez, A., Guja, C., MItrakou, A., Lalic, N., Tankova, T., Czupryniak, L., Tabak, A. G., Prazny, M., Martinka, E., & Smircic-Duvnjak, L. (2020). Diabetes Therapy, 11(2), 387-409. https://doi.org/10.1007/s13300-019-00743-7
Jendle, J. H., Adolfsson, P., & Pollock, N. W. (2020). Recreational diving in persons with type 1 and type 2 diabetes: Advancing capabilities and recommendations. Diving and Hyperbaric Medicine, 50(2), 135-143. https://doi.org/10.28920/dhm50.2.135-143
Jonas, D. E., Crotty, K., Yun, J. D. Y., Middleton, J. D., Feltner, C., Taylor-Phillips, S., Barclay, C., Dotson, A., Baker, C., Balio, C. P., Voisin, C. E., & Harris, R. P. (2021). Screening for prediabetes and type 2 diabetes: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA, 326(8), 744-760. https://doi.org/10.1001/jama.2021.10403
Khardori, R. (2022). Type 1 diabetes mellitus. https://emedicine.medscape.com/article/117739-overview#a5
Kochar, I. S., & Jain, R. (2021). Pancreas transplant in type 1 diabetes mellitus: The emerging role of islet cell transplant. Annals of Pediatric Endocrinology & Metabolism, 26(2), 86-91. https://doi.org/10.6065/apem.2142012.006
Labib, A., Rosen, J., & Yosipovitch, G. (2022). Skin manifestations of diabetes mellitus. Endotext [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK481900
Levitsky, L. L., & Misra, M. (2023). Epidemiology, presentation, and diagnosis of type 1 diabetes mellitus in children and adolescents. UpToDate. Retrieved June 22, 2023, from https://www.uptodate.com/contents/epidemiology-presentation-and-diagnosis-of-type-1-diabetes-mellitus-in-children-and-adolescents
Lightner, D. J., Gomelsky, A., Souter, L., & Vasavada, S. P. (2019). Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment 2019. Journal of Urology, 202(3), 558-563. https://doi.org/10.1097/JU.0000000000000309
Lucier, J., & Weinstock, R. S. (2023). Type 1 diabetes. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK507713
Mathew, P., & Thoppil, D. (2022). Hypoglycemia. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK534841
Mathew, T. K., Zubair, M., & Tadi, P. (2023). Blood glucose monitoring. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK555976
Mehta, S. (2022). Diabetic retinopathy. Merck Manual Professional Version. https://www.merckmanuals.com/professional/eye-disorders/retinal-disorders/diabetic-retinopathy
Mosenzon, O., Cheng, A. Y. Y., Rabinstein, A. A., & Sacco, S. (2023). Diabetes and stroke: What are the connections? Journal of Stroke, 25(1), 26-38. https://doi.org/10.5853/jos.2022.02306
Mouri, M., & Badireddy, M. (2023). Hyperglycemia. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK430900
Mutanga, J. N., Nukala, U., Messan, M. R., Yogurtcu, O. N., McCormick, Q., Sauna, Z. E., Whitaker, B. I., Forshee, R. A., & Yang, H. (2023). A retrospective review of Center for Biologics Evaluation and Research Advisory Committee meetings in the context of the FDA's benefit-risk framework. American Association of Pharmaceutical Scientists, 25(1), 24. https://doi.org/10.1208/s12248-023-00789-3
National Center for Complementary and Integrative Health. (2020). Yohimbe. US Department of Health and Human Services. https://www.nccih.nih.gov/health/yohimbe
National Institute of Diabetes and Digestive and Kidney Diseases. (n.d.-a). Close-up of beta cells in pancreatic islet - labeled [Image]. Retrieved July 27, 2023, from https://www.niddk.nih.gov/news/media-library/18115
National Institute of Diabetes and Digestive and Kidney Diseases. (n.d.-b). Cross section of an eye showing three stages of diabetes damage [Image]. Retrieved July 27, 2023, from https://www.niddk.nih.gov/news/media-library/17833
National Institute of Diabetes and Digestive and Kidney Diseases. (n.d.-c). Pancreas responds to low or high blood glucose levels [Image]. Retrieved July 27, 2023, from https://www.niddk.nih.gov/news/media-library/17969
National Institute of Diabetes and Digestive and Kidney Diseases. (2016). Diabetes diet, eating, & physical activity. https://www.niddk.nih.gov/health-information/diabetes/overview/diet-eating-physical-activity
National Institute of Diabetes and Digestive and Kidney Diseases. (2018a). Diabetes, sexual and bladder problems. https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-problems/sexual-bladder-problems?dkrd=/health-information/diabetes/overview/preventing-problems/sexual-urologic-problems
National Institute of Diabetes and Digestive and Kidney Diseases. (2018b). Gastroparesis. https://www.niddk.nih.gov/health-information/digestive-diseases/gastroparesis
National Institute of Diabetes and Digestive and Kidney Diseases. (2021a). Artificial pancreas. https://www.niddk.nih.gov/health-information/diabetes/overview/managing-diabetes/artificial-pancreas
National Institute of Diabetes and Digestive and Kidney Diseases. (2021b). Bladder control problems (urinary incontinence). https://www.niddk.nih.gov/health-information/urologic-diseases/bladder-control-problems
O'Neal, T. B., & Luther, E. E. (2023). Dawn phenomenon. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK430893
Paschou, S. A., & Papanas, N. (2019). Type 2 diabetes mellitus and menopausal hormone therapy: An update. Diabetes Therapy, 10, 2313-2320. https://doi.org/10.1007/s13300-019-00695-y
Pullen, L. C. (2021). Islet cell transplantation hits a milestone. American Journal of Transplantation, 21(8), 2625-2626. https://doi.org/10.1111/ajt.16039
Quan, D. (2021). Diabetic neuropathy medication. https://emedicine.medscape.com/article/1170337-medication#2
Redondo, M. J., Steck, A. K., & Pugliese, A. (2018). Genetics of type 1 diabetes. Pediatric Diabetes, 19(3), 346-353. https://doi.org/10.1111/pedi.12597
Regufe, V. M. G., Pinto, C. M. C. B., & Perez, P. M. V. H. C. (2020). Metabolic syndrome in type 2 diabetic patients: A review of current evidence. Porto Biomedical Journal, 5(6), e101. https://doi.org/10.1097/j.pbj.0000000000000101
Rewers, M., Stene, L. C., & Norris, J. M. (2018). Risk factors for type 1 diabetes. In C. C. Cowie, S. S. Casagrande, & A. Menke (Eds.), Diabetes in America (3rd ed., chapter 11). https://www.ncbi.nlm.nih.gov/books/NBK567965
Reyhanoglu, G., & Rehman, A. (2023). Somogyi phenomenon. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK551525
Rubin, M. (2022). Mononeuropathies. Merck Manual Professional Version. https://www.merckmanuals.com/professional/neurologic-disorders/peripheral-nervous-system-and-motor-unit-disorders/mononeuropathies?query=Mononeuropathy
Sapra, A., & Bhandari, P. (2023). Diabetes. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK551501
Shindel, A. W., & Lue, T. F. (2021). Sexual dysfunction in diabetes. Endotext [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK279101
Subramanian, S., & Baidal, D. (2021). The management of type 1 diabetes. Endotext [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK279114
Thornton, K., Chervenak, J., & Neal-Perry, G. (2015). Menopause and sexuality. Endocrinology and Metabolism Clinics of North America, 44(3), 649-661. https://doi.org/10.1016/j.ecl.2015.05.009
US Preventive Services Task Force. (2021a). Screening for gestational diabetes: US Preventive Services Task Force Recommendation Statement. JAMA, 326(6), 531-538. https://doi.org/10.1001/jama.2021.11922
US Preventive Services Task Force. (2021b). Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force Recommendation Statement. JAMA, 326(8), 736-743. https://doi.org/10.1001/jama.2021.12531
Van Cauwenberghe, J., Enzlin, P., Nefs, G., Ruige, J., Hendrieckx, C., De Block, C., & Pouwer, F. (2022). Prevalence of and risk factors for sexual dysfunctions in adults with type 1 or type 2 diabetes: Results from diabetes MILES-Flanders. Diabetic Medicine, 39(e14676). https://doi.org/10.1111/dme.14676
Whelton, P. K., Carey, R. M., Aronow, W. S., Casey, D. E., Collins, K. J., Himmelfarb, C. D., DePalma, S. M., Gidding, S., Jamerson, K. A., Jones, D. W., MacLaughlin, E. J., Muntner, P., Ovbiagele, B., Smith, S. C., Spencer, C. C., Stafford, R. S., Taler, S. J., Thomas, R. J., Williams, K. A., . . . Wright, J. T. (2017). 2017 Clinical practice guideline: ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension, 71, e13-e115. https://doi.org/10.1161/HYP.0000000000000065
Wood, J., & Peters, A. (2018). The type 1 diabetes self-care manual. The American Diabetes Association. http://main.diabetes.org/dorg/PDFs/living-with-diabetes/T1DSelfCareManual.pdf
Woods, A. D. (2023). Nursing 2023 drug handbook (43rd ed.). Wolters Kluwer.
Young, C. F., Moussa, M., & Shubrook, J. H. (2020). Diabetic gastroparesis: A review. Diabetes Spectrum, 33(3), 290-297. https://doi.org/10.2337/ds19-0062
Zaino, B., Goel, R., Devaragudi, S., Prakash, A., Vaghamashi, Y., Sethi, Y., Patel, N., & Kaka, N. (2023). Diabetic neuropathy: Pathogenesis and evolving principles of management. Disease-a-Month. https://doi.org/10.1016/j.disamonth.2023.101582