About this course:
This course discusses early mobilization (EM) of mechanically ventilated patients, including the background, pathophysiology, indications for, and complications of mechanical ventilation (MV) and the safety considerations, screening process, and implementation strategies needed to optimize patient outcomes.
Course preview
Early Mobilization for Mechanically Ventilated Patients
This course discusses early mobilization (EM) of mechanically ventilated patients, including the background, pathophysiology, indications for, and complications of mechanical ventilation (MV) and the safety considerations, screening process, and implementation strategies needed to optimize patient outcomes.
At the completion of this course, learners will be prepared to:
- Discuss the background and incidence of MV in critically ill patients.
- Describe the pathophysiology of MV for the respiratory system.
- Identify indications for MV in critically ill patients.
- Explore various complications of MV and their effects on patient outcomes.
- Describe strategies to prevent complications of MV, including EM.
- Discuss the safety and effectiveness of EM, the screening process for appropriate patients, and strategies to assist with the implementation of EM programs in an intensive care unit (ICU).
Health care providers (HCPs) are responsible for offering high-quality, evidence-based care to optimize patient outcomes. As new treatments emerge, people are living longer, healthier lives. As the US population continues to age, more people are living with chronic health conditions. As more people manage these complex chronic conditions, HCPs will see increasing numbers of patients admitted to critical care units (Dirkes & Kozlowski, 2019). Traditionally, HCPs working with critically ill patients have focused on stabilizing the immediate life-threatening cardiopulmonary symptoms. As survival from critical illness has improved, the medical focus has shifted to preventing the sequelae of critical illness, including neuromuscular weakness, cognitive impairment, and psychological disorders (Physiopedia, n.d.-a; The TEAM Study Investigators and the ANZICS Clinical Trials Group, 2022). Critically ill patients are often admitted to intensive care units (ICUs) so that HCPs can manage physiological responses to illness effectively. Mechanical ventilation (MV) is frequently used for critically ill patients requiring airway protection or respiratory support. However, hemodynamic instability, altered sleep patterns, vascular access devices (VAD), and sedation can limit the mobility of these patients (Doiron et al., 2018). Early mobilization (EM) of critically ill patients, including those receiving MV, can decrease the weakness and deconditioning associated with critical illnesses (Physiopedia, n.d.-a; The TEAM Study Investigators and the ANZICS Clinical Trials Group, 2022).
Background
The Centers for Disease Control and Prevention (CDC, 2024a, 2024c) defines chronic diseases as conditions that last more than 1 year and require ongoing medical attention and/or limit activities of daily living. Chronic disease is the leading cause of death and disability in the US. An estimated 6 out of 10 adults in the US have at least one chronic disease, and 4 out of 10 adults have two or more chronic diseases. Chronic conditions, such as heart disease, cancer, chronic lung disease, diabetes mellitus (DM), Alzheimer's disease, and chronic kidney disease (CKD), significantly contribute to the $4.5 trillion spent on US health care costs annually. The current life expectancy for adults in the US is 77.5 years (CDC, 2024a, 2024b). As the population lives longer with complex chronic conditions, more advanced medical treatments will be necessary. More than 5 million patients are admitted to ICUs in the US annually for intensive or invasive monitoring, including airway, breathing, or circulation support; stabilization of acute or life-threatening medical problems; and comprehensive management of injury or illness. Although the ICU patient population is heterogeneous, the most common indications for admission include cardiac, respiratory, and neurological conditions. Respiratory failure with ventilator support is among the top five ICU admissions for adults. In addition, the most common technological support required in the ICU is MV, accounting for 20% to 40% of admissions in the US. Annual critical care costs have increased by 92% between 2000 and 2010, rising from $56 billion to $108 billion. ICU costs per day were estimated to be $4,300 in 2010, representing a 61% increase since 2000. Although more recent data on critical care costs are not reported in the literature, it is expected that health care expenditures will rise 5.4% annually from 2022 to 2031 (Society of Critical Care Medicine [SCCM], 2024).
MV is a common cause of ICU admission for patients requiring airway protection or respiratory support (Mora Carpio & Mora, 2023). MV is defined as delivering positive pressure to the lungs through a tracheostomy or endotracheal tube (Hyzy & McSparron, 2024). By performing the work of breathing and gas exchange, MV can fully or partially replace the functions of spontaneous breathing for patients who have respiratory failure. During MV, a predetermined air mixture (oxygen [O2] and other gases) is forced into the central airways and travels into the alveoli. As a result, the lungs inflate, causing intra-alveolar pressure to increase. The ventilator stops forcing air into the central airways when a termination signal occurs, usually from increased flow or pressure. Expiration passively occurs as the central airway pressure decreases, with air flowing from the higher-pressure alveoli to the lower-pressure central airways (Hyzy & McSparron, 2024). HCPs caring for patients requiring MV must understand the following ventilator-related concepts (Mora Caprio & Mora, 2023):
- Ventilation is the movement and exchange of gases (O2 and carbon dioxide [CO2]) between the lungs and the air. A ventilator forces O2 into the lungs, and CO2 is removed from the body during exhalation. In a patient on MV, the CO2 in the blood can be modified by changing the tidal volume (TV) or respiratory rate (RR).
- Oxygenation of mechanically ventilated patients, which boosts the O2 supply to the lungs, can be achieved by increasing the fraction of inspired oxygen (FiO2) or the positive end-expiratory pressure (PEEP).
- TV is the volume of air moved in and out of the lungs with each respiratory cycle.
- PEEP refers to the positive pressure applied by the ventilator at the end of each respiratory cycle. In mechanically ventilated patients, this pressure remains greater than the atmospheric pressure in order to prevent the alveoli from collapsing.
- FiO2 is the percentage of O2 in the air mixture that the mechanical ventilator delivers to a patient.
The Centers for Medicare and Medicaid Services (CMS) define prolonged mechanical ventilation (PMV) as more than 21 days of MV for at least 6 hours per day (King Han, 2023). An estimated 300,000 patients in the US require MV each year (National Healthcare Safety Network[NHSN], 2024). Of these patients, between 4% and 13% require PMV, which equates to 7,250 and 11,400 patients undergoing PMV at any time. PMV is associated with increased length of stay (LOS), health care costs, ventilator-associated events (VAEs), morbidity, and mortality. Therefore, the prevention of PMV and VAEs is essential for HCPs to optimize patient outcomes (King Han, 2023).
Indications for MV
A mechanical ventilator is used to decrease the work of breathing until patients can resume normal breathing on their own. A ventilator ensures that a patient receives adequate O2 to be used by the tissues and also removes CO2. By partially or fully assisting...
...purchase below to continue the course
Pathophysiology
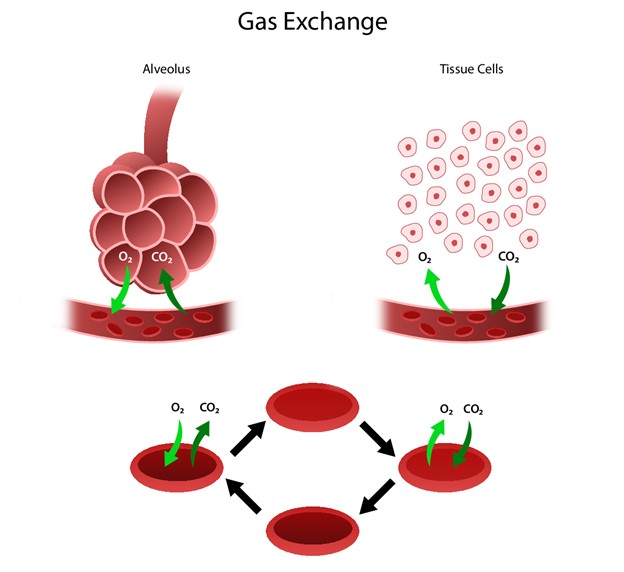
The respiratory system facilitates life-sustaining processes, including O2 transport, respiration, ventilation, and gas exchange. The body's cells rely on the oxidation of carbohydrates, fats, and proteins to produce energy. Without a continuous supply of O2, cells in the brain, heart, and other essential organs cannot survive. O2 is transported to, and CO2 is removed from, the circulating blood through the thin walls of the capillaries. O2 diffuses through capillary walls to the interstitial fluid and eventually to the cells. CO2 diffuses in the opposite direction, going from the cells to the blood. After these tissue capillary exchanges, blood enters the systemic venous circulation and travels to the pulmonary circulation. The O2 concentration in the alveoli is higher than the concentration in the blood. Therefore, O2 diffuses from the alveoli to the blood. Similarly, CO2 has a higher concentration in the blood than in the alveoli, so it diffuses from the blood to the alveoli (see Figure 1; Hinkle et al., 2021).
Figure 1
Human Gas Exchange
Ventilation requires movement of the walls of the thoracic cage and the diaphragm. The movement of the thoracic cage and diaphragm alternate by increasing and decreasing the chest's capacity. When the chest capacity increases, air enters the trachea and passes through the bronchi and bronchioles and into the alveoli to inflate the lungs (inspiration). As the thoracic cavity expands, the pressure inside the thorax is lower than the atmospheric pressure, and air flows from a region of higher pressure to lower pressure. During expiration, the diaphragm relaxes and the lungs recoil, decreasing the size of the thoracic cavity. As the pressure in the alveoli increases, air flows from the lungs to the atmosphere. The inspiratory phase of respiration requires active energy, while the expiratory phase is passive. In addition to air pressure variances, airway resistance can also impact ventilation. The size or diameter of the airway, lung volumes, and airflow velocity determine airway resistance. Any process that changes the diameter of the airway will affect airway resistance and the rate of airflow. With increased airway resistance, a more significant respiratory effort will be required to achieve normal ventilation (Hinkle & Cheever, 2021).
Normal respiratory function works as a negative pressure system. This negative intrathoracic pressure decreases the right atrial (RA) pressure, which creates a pulling effect on the inferior vena cava (IVC), resulting in increased venous return. Conversely, MV alters normal respiratory function by applying positive-pressure ventilation. MV pushes air into the upper airways and alveoli, creating positive pressure in the thoracic cavity. This positive intrathoracic pressure increases the RA pressure and decreases venous return, thereby reducing preload (the force that stretches the cardiac muscle before constriction). With less blood reaching the left ventricle, cardiac output decreases, and mean arterial pressure (MAP) drops. In addition, the positive pressure generated by MV can significantly reduce the work of breathing. With a decrease in work of breathing, blood flow can be redistributed from the respiratory muscles to more critical organs. Reducing how hard the respiratory muscles work limits CO2 and lactate generation, improving acidosis (see Figure 2; Mora Carpio & Mora, 2023). By reducing the burden on the respiratory muscles, MV may induce respiratory muscle and diaphragmatic weakness. This respiratory muscle weakness is a significant predictor of PMV (King Han, 2023).
Figure 2
Biology of Ventilation

(Lutz, 2020)
Consequences of Mechanical Ventilation
Patients requiring MV are at high risk for complications and poor outcomes, including death. Barotrauma, pulmonary embolism (PE), sepsis, acute respiratory distress syndrome (ARDS), and ventilator-associated pneumonia (VAP) are VAEs that can affect patients receiving MV. These complications can result in PMV, prolonged ICU stays, increased health care costs, and high mortality. Mortality rates for patients who have an acute lung injury (ALI) requiring MV range from 24% in persons 15 to 19 years to 60% for patients 85 years and older (NHSN, 2024). Before 2013, the NHSN surveillance for VAEs was limited to VAPs. However, accurate VAP surveillance was a challenge due to inconsistent VAP definitions, with many requiring radiographic findings of pneumonia. The subjectivity and variability of chest radiographic technique, interpretation, and reporting make this imaging inappropriate for VAP diagnosis. Another limitation of the VAP surveillance definition was the reliance on specific clinical signs and symptoms, which are subjective and inconsistently documented. In 2013, the NHSN implemented the VAE surveillance definition algorithm based on objective and streamlined criteria to identify a broad range of conditions or complications occurring in mechanically ventilated patients (NHSN, 2024). The CDC defined a VAE as a sustained increase in ventilator support following a period of stable or decreasing ventilator support. This definition broadened the focus from pneumonia alone to encompass all major causes of respiratory deterioration in ventilated patients (pneumonia plus pulmonary edema, ARDS, PE, atelectasis; Klompas, 2019; Patel, 2024).
In 2016, VAE rates for critical care units nationwide were 6.8 events per 1,000 ventilator days. These rates varied substantially by ICU type, with higher rates in trauma, surgery, and neurological units and lower rates in medical and cardiac units. VAE rates were also higher in teaching hospitals compared to non-teaching hospitals. Although patients are at risk for VAEs until extubation occurs, the highest risk was found in the first 2 weeks of MV (specifically days 3 to 7). VAEs are divided into two subcategories to distinguish VAPs from other ventilator-associated complications. Infection-related ventilator-associated complications (IVAC) are defined as the presence of an abnormal temperature or white blood cell (WBC) count and at least 4 days of newly prescribed antibiotics starting within 2 days of VAE onset. Most VAEs are triggered by one of four clinical events: pneumonia (25% to 40% of cases), ARDS (5% to 20% of cases), atelectasis (10% to 15% of cases), and fluid overload (pulmonary edema; 15% to 50% of cases). Approximately one-third of VAEs meet the IVAC criteria. The overall mortality rate for VAEs is 31%, and those patients developing VAEs are twice as likely to die compared to patients who did not develop VAEs. Thus, the prevention of VAEs is critical to optimizing outcomes for patients receiving MV. Strategies for preventing VAEs can include elevating the head of the bed, oral care with chlorhexidine, spontaneous awakening and breathing trials, and early mobility (Klompas, 2019).
Ventilator-Associated Pneumonia
VAP is a lung infection that develops in mechanically ventilated patients when pathogens enter through the endotracheal tube and reach the patient's lungs (CDC, 2024d). VAP is a type of hospital-acquired pneumonia (HAP) that develops after 48 hours of MV (Kollef, 2023). Only 40% of VAPs meet the criteria for VAEs, with 60% clinically diagnosed without sustained increases in ventilator support (Klompas, 2019). According to the NHSN (2024), VAP rates have steadily declined in the US between 2006 and 2012, from 3.1 to 0.9 per 1,000 ventilator days in medical ICUs and from 5.2 to 2 per 1,000 ventilator days in surgical ICUs. It is unclear if this data represents a true decline in VAP rates or stricter application of the VAP criteria. However, VAP is associated with extended hospital stays, PMV, and significant health care costs ($40,000 per patient) and is an important consideration for HCPs caring for patients requiring MV (Klompas, 2023). Various pathogens may cause VAP and can be polymicrobial. Some of the most common aerobic gram-negative bacilli include Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa, and gram-positive cocci include Staphylococcus aureus and Streptococcus (Klompas, 2023). Therefore, HCPs must be acutely aware of the clinical features associated with VAP. Most patients who have VAP present with sudden or gradual onset of the following clinical features:
- Dyspnea (although many patients requiring MV may be unable to report symptoms due to sedation or severity of illness)
- Tachypnea, increased or purulent sections, rhonchi, crackles, reduced breath sounds, and fever
- Ventilator mechanics that show reduced total volume and increased inspiratory pressures
- Laboratory findings that show leukocytosis and worsening hypoxemia
- Imaging results that show new or worsening infiltrate on a chest x-ray (Kollef, 2023)
As isolated findings, none of these clinical features can be sensitive for or specific to the diagnosis of VAP. Therefore, a chest radiograph should be performed for all patients who are suspected to have VAP In order to evaluate a new or progressive pulmonary infiltrate. A VAP diagnosis can be made by identifying a pulmonary infiltrate on imaging with clinical findings of infection, as outlined previously (Kollef, 2023).
Prolonged Mechanical Ventilation
PMV increases the risk of pneumonia, barotrauma, tracheal injuries, musculoskeletal deconditioning, and mortality. Strategies such as EM of patients on MV can help prevent PMV and facilitate the weaning process. Prolonged ventilatory dependence can stem from many factors, including respiratory insufficiency, reduced ventilatory capacity, and cardiovascular insufficiency. Respiratory insufficiency occurs when there is an imbalance between respiratory pump capacity and demands. In addition, PMV can lead to diaphragmatic weakness and atrophy, especially when accompanied by passive modes of ventilation. Other factors contributing to respiratory muscle weakness include malnutrition, immobility, excessive steroids, sedative medications, and a systemic inflammatory response (SIR) associated with sepsis. Cardiovascular insufficiency (heart failure [HF]) can also increase the risk of PMV. When a patient transitions from MV to spontaneous breathing, there is a loss of positive intrathoracic pressure. This shift in pressure raises venous return in the right ventricle, increasing preload and afterload. This greater cardiac workload can raise myocardial O2 demand in patients who have coronary artery disease, resulting in ischemia (Fadila et al., 2022; Huang et al., 2022). The following risk factors can increase the risk of PMV:
- History of COPD
- History of restrictive pulmonary disease
- Neuromuscular disorders
- ICU admission due to pneumonia, ARDS, head trauma, and post-operative intracerebral hemorrhage
- Abnormal arterial CO2, serum blood urea nitrogen (BUN), serum creatinine, arterial PH, WBC count, or body temperature on the first ICU day
- Age 85 years and older
- Extended inpatient length of stay before ICU admission (Fadila et al., 2022; Huang et al., 2022; King Han, 2023)
ICU-Acquired Weakness (ICUAW)
Neuromuscular weakness is a phenomenon that affects patients who have critical illnesses. ICUAW occurs more frequently due to improved survival rates among patients who have multi-organ failure and sepsis. ICUAW refers to patients who have clinically detected weakness with no determined cause other than their critical illness (Lacomis, 2023). Neuromuscular weakness can occur secondary to critical illness, prolonged bed rest, and immobility, leading to impaired physical function. Approximately 50% of ICU patients experience physical impairment, and at least 50% cannot return to pre-illness activity levels (Agency for Healthcare Research and Quality [AHRQ], 2017a). In addition, ICUAW is associated with PMV, sepsis, systemic inflammatory response syndrome (SIRS), multi-organ failure, VAP, and hyperglycemia. Mechanically ventilated patients and those who have a critical illness can sustain a loss of muscle mass within a few days of ICU admission. These patients are more likely to experience poor clinical outcomes, higher health-related costs, poor quality of life (QOL), and persistent weakness for months to years after ICU admission. Patients who have ongoing neuromuscular weakness have significantly higher mortality rates a year after admission to the ICU (Doiron et al., 2018). ICUAW is estimated to affect 30% to 50% of critically ill patients, with as many as 67% of these patients affected by sepsis (Cheung et al., 2021). Females and patients with low muscle mass, malnutrition, and severe illness are more likely to experience ICUAW (Physiopedia, n.d.-b).
ICUAW is characterized by symmetrical, flaccid limb weakness with diaphragmatic involvement. Facial and ocular muscles remain intact (Cheung et al., 2021; Vanhorebeek et al., 2020). The pathogenesis of ICUAW is not entirely understood, making its prevention and treatment complicated. However, neuromuscular weakness in the ICU may often be related to muscle wasting or atrophy, critical illness myopathy (CIM), and critical illness polyneuropathy (CIP, Lacomis, 2023). Critical illness polyneuromyopathy (CIPNM) involves the combination of both CIP and CIM. Although CIP and CIM are often discussed within the context of ICUAW, there are differences in their pathophysiology, prognosis, and management approaches. Many studies have found CIP to be associated with significantly worse clinical outcomes than CIM. Multiple mechanisms can lead to critically ill patients developing ICUAW, including the following:
- Reduced excitability of muscles and nerves
- Death of peripheral nerve axons
- Myosin loss, myofiber atrophy, and death
- Bioenergetic failure
- Altered calcium and sodium (ionic) regulation
- Neuromuscular transmission dysfunction
- Increases in systemic inflammation
- Altered catabolism-to-anabolism ratio (Cheung et al., 2021; Vanhorebeek et al., 2020)
These factors will be discussed next within the context of CIM and CIP.
Critical Illness Myopathy
CIM is a common cause of ICUAW, delayed recovery, and PMV. This disorder is also known as acute quadriplegic myopathy, thick-filament myopathy, ICU myopathy, critical care myopathy, and acute necrotizing myopathy of intensive care (Z'Graggen & Tankisi, 2020). Although the underlying mechanisms of CIM are not entirely understood, muscle membrane inexcitability, myosin loss, and muscle necrosis are all involved in its development (Lacomis, 2023). Structurally, CIM is characterized by the selective loss of myosin, which causes myofiber atrophy and death. Some underlying causes of CIM include systemic inflammation, metabolism, microcirculation at the muscle level, dysfunction of the membrane/ion channels, and structural skeletal muscle changes. Inflammation, which is related to the significant increase in proinflammatory cytokines in critically ill patients, impacts the development of weakness. In addition, patients who have CIM experience a selective loss of myosin within the myofibers. This preferential myosin loss may be related to prolonged immobilization (over 5 days) and compounded by diaphragmatic myosin loss from MV. The hypothalamic-pituitary axis plays a critical role in regulating metabolic homeostasis during stressful periods (growth hormone and cortisol). In critically ill patients, the release of growth hormone is suppressed, which contributes to muscle wasting. In addition, excessive cortisol exerts catabolic effects on skeletal muscles, which immobility exacerbates. Finally, patients who have CIM may experience sodium channel dysfunction, which results in sarcolemma (the sheath that surrounds the fibers of the skeletal muscles) inexcitability, directly impacting muscle weakness (Cheung et al., 2021).
Critical Illness Polyneuropathy
CIP is a common complication of severe sepsis and may be a neurologic manifestation of SIRS (Lacomis, 2023). CIP is defined as weakness related to the dysfunction and degradation in multiple peripheral nerves (without the demyelinating component of other acute neurological conditions). Similar to CIM, many proposed mechanisms contribute to the development of CIP. One proposed mechanism of CIP is an alteration of the microvasculature of the axons of peripheral nerves. Increased permeability to the vasa nervorum results from increased E-selectin expression (a membrane-activation marker found in patients who have CIP). The resultant transmigration of immune cells into nerve tissue, through the release of inflammatory mediators, represents an inflammatory response. The resulting edema may induce hypoxia, which leads to an increase in reactive oxygen species (ROS) formation and impaired energy generation. These energy deficits can lead to axonal degeneration (Cheung et al., 2021; Lacomis, 2023).
Hyperglycemia may also directly affect axons, influencing mitochondrial function negatively. Although the underlying mechanism remains unclear, two possible theories explain why hyperglycemia is more toxic in ICU patients than in non-critically ill patients. The first theory is that patients who have critical illness experience cellular glucose overload, which has a toxic effect on axons. This glucose overload upregulates glucose transporters and various tissue types, including cytokines, angiotensin II, and neurons. Therefore, the stress response overrides normal glucose homeostatic measures, allowing for more rapid, toxic glucose overload. The second theory hypothesizes that increased glucose levels increase ROS generation. This increase in ROS, taking the form of superoxide, is produced by glycolysis and oxidative phosphorylation processes. The creation of superoxide molecules overwhelms the cells' native ROS-protective mechanisms, allowing unneutralized ROS to combine with nitric oxide, generating peroxynitrites. Peroxynitrites can suppress the mitochondrial electron transport chain, resulting in cell death (Cheung et al., 2021; Lacomis, 2023).
Risk Factors for ICUAW
Identifying risk factors for developing ICUAW can assist HCPs in the prevention and management of these complications. Sepsis, SIRS, and multi-organ failure are common and well-supported risk factors for critically ill patients to develop ICUAW. In addition, other risk factors include immobility, corticosteroids, hyperglycemia, prolonged sedation, neuromuscular blockades, MV duration, catecholamine administration, and renal replacement therapy (Cheung et al., 2021; Hashem, Parker, et al., 2016; Kho & Connolly, 2023; Lacomis, 2023).
Bed Rest
For critically ill patients, bed rest and immobility can result in a substantial loss of muscle mass and strength (Hashem, Parker, et al., 2016). During bed rest, skeletal muscles are activated less often and for shorter periods. This underutilization of muscles triggers a cascade of responses, including slowed protein synthesis, accelerated proteolysis, and increased apoptosis. These mechanisms alter the proportion of slow- and fast-twitch muscle fibers, contractility, and aerobic capacity resulting in muscle weakness. It is estimated that after about 2 weeks of immobilization, young, healthy adults can experience 5% to 9% quadriceps muscle mass loss and a 20% to 27% decrease in quadriceps muscle strength. In addition, older adults can experience a three- to six-fold greater rate of muscle loss. In the past, bed rest was a routine practice for patients receiving MV in the ICU, with these patients receiving limited out-of-bed mobilization. Muscle biopsies from mechanically ventilated patients show signs of inflammation, necrosis, and replacement of muscle fibers with adipose and connective tissue. To prevent these consequences of immobility, health care institutions have moved away from bed rest to EM for patients requiring MV (Damluji et al., 2019; Hashem, Parker, et al., 2016).
In a prospective longitudinal study, Fan and colleagues (2014) followed 222 survivors of ARDS for two years. After adjusting for baseline characteristics and risk factors for muscle weakness, the duration of bed rest was the only strong, independent, and consistent factor associated with muscle weakness. Follow-up assessments occurred at 3, 6, 12, and 24 months. The researchers found a 3% to 11% relative decrease in muscle strength for each additional day of bed rest in the ICU. Similarly, Needham and colleagues (2014) conducted a prospective longitudinal study following 203 survivors of ARDS for 12 months after discharge. After adjusting for baseline characteristics and other risk factors for muscle weakness, the researchers found that ICU LOS had a significant and independent association with decreased muscle strength. LOS in the ICU was an approximation of bed rest because 75% of patients did not routinely receive mobilization while admitted.
Corticosteroids
Corticosteroid therapy is often utilized for critically ill patients and those requiring MV because of its anti-inflammatory and antifibrotic effects. Corticosteroid therapy causes changes in specific gene expression, resulting in the inhibition of protein synthesis, which promotes muscle wasting. Based on these mechanisms, corticosteroid use is a risk factor for the development of ICUAW. In a systematic review exploring the relationship between corticosteroid use and ICUAW in ICU patients, Yang and colleagues (2018) found a significant association between corticosteroid use and ICUAW. The researchers reviewed 18 studies with a total of 2,387 patients and found no significant association between the use of corticosteroids and ICUAW for those with sepsis. However, the researchers did find a significant association between corticosteroids and ICUAW in mechanically ventilated patients. They concluded that the effect of corticosteroid therapy on ICUAW is complex and may depend on the duration and cumulative dosage of prescribed corticosteroids. HCPs should consider limiting exposure to or shortening the duration of corticosteroid administration to decrease the incidence of ICUAW (Yang et al., 2018).
Clinical Assessment of ICUAW
HCPs caring for critically ill patients or patients requiring MV must frequently monitor for signs of ICUAW. The classic manifestations of ICUAW are flaccid quadriparesis and neuromuscular respiratory failure. ICUAW is typically diagnosed during the recovery period from critical illness when prolonged or failed weaning from MV or profound weakness in a conscious patient is identified (Cheung et al., 2021; Kramer, 2017; Taylor, 2021). Early identification of ICUAW clinical features can be challenging due to sedation, encephalopathy, pharmacologic paralysis, or the severity of illness. For an unconscious patient, early clinical signs include a limited or absent limb response to painful stimuli, with facial grimacing indicating intact sensory function. Muscular changes can occur as early as 24 hours after ICU admission but appear to begin acutely after encephalopathy improves and sedation and neuromuscular blockades are discontinued. HCPs must closely monitor acute changes in critically ill patients while also considering the impact of underlying health conditions. If a patient is conscious, HCPs should assess for a prior history of weakness, the new onset of weakness, the pattern of the weakness, difficulty swallowing, newly started medications, and the presence or lack of sensory complaints. For patients on MV, neuromuscular respiratory weakness can present as low TV or the need for high-pressure support on the spontaneous mode of MV. Extubated patients who have neuromuscular respiratory weakness may experience tachypnea, tachycardia, diaphoresis, interrupted speech, or orthopnea (Andreae & Hong, 2024; Kramer, 2017; Lacomis, 2023).
Clinical Assessment of CIM and CIP
Clinical features common to both CIM and CIP include symmetric, flaccid limb weakness and respiratory muscle weakness. However, extraocular and facial muscles are usually not involved. CIM often begins within several days of ICU admission, but unconscious patients can experience delayed symptom identification. The most common clinical features for CIM include flaccid quadriparesis that may affect proximal more than distal muscles and failure to wean from MV. Sensation should remain intact. CIP usually affects patients who have been admitted to the ICU for over 1 week. As discussed previously, some clinical features of CIP may be similar to those in CIM. For example, patients who have CIP have difficulty weaning from MV (Kramer, 2017; Lacomis, 2023; Taylor, 2021). Clinical manifestations of CIP include sensorimotor polyneuropathy characterized by the following:
- Reduced or absent deep tendon reflexes
- Limb muscle weakness and atrophy, which are worse distally than proximally
- Peripheral sensation loss to light touch and pinprick
- Relative preservation of cranial nerve function (Kramer, 2017; Lacomis, 2023)
CIPNM has overlapping clinical features of CIM and CIP, including symmetric weakness of all four limbs (typically affecting proximal more than distal muscles), peripheral sensory loss, and reduced or absent deep tendon reflexes (Lacomis, 2023; Taylor, 2021).
Evaluation for ICUAW, CIM, and CIP
For critically ill patients and patients receiving MV, HCPs must diligently monitor for signs and symptoms of ICUAW. Physical examinations can be challenging because of the severity of the illness. Muscle strength testing requires patients to be alert and cooperative during the examination, which may not be possible for patients experiencing encephalopathy or receiving sedation or paralytic medications. HCPs should assess for voluntary movement in all four extremities. In addition, muscle strength can be tested manually in each extremity and graded using the Medical Research Council (MRC) scale. The MRC scale grades muscle strength by the following:
- 0: No contraction
- 1: Flicker or trace of contraction
- 2: Active movement with gravity eliminated
- 3: Active movement against gravity
- 4: Active movement against gravity with resistance
- 5: Full strength
HCPs should use the MRC to test the strength of three movements in each arm (shoulder abduction, elbow flexion, and wrist extension) and each leg (hip flexion, knee extension, and ankle dorsiflexion), yielding a maximum combined score of 60. A combined score below 48 is considered diagnostic of ICUAW. A mean MRC score below 4 per muscle group is also considered diagnostic for ICUAW. The main limitation of using the MRC scale is the ability to reliably exclude other causes of generalized weakness, such as a central nervous system (CNS) problem (Kramer, 2017; Lacomis, 2023; Taylor, 2021).
CIM can be distinguished from CIP by assessing sensory function. In patients with CIM, sensory function is preserved. However, similar to muscle strength, assessing sensory function can be challenging for sedated or encephalopathic patients. For patients whose neurological assessment of weakness is unreliable, neuroimaging (magnetic resonance image [MRI]) is recommended to evaluate for CNS lesions (Lacomis, 2023). The gold standard for CIM and CIP includes electrodiagnostic testing with electromyography (EMG) and nerve conduction studies (NCS). EMG is a diagnostic procedure to assess the health of muscles and the motor neurons (nerve cells that control muscles). Motor neurons send electrical signals to the muscles, causing contraction. An EMG uses electrodes to translate these electrical signals into graphs or numerical values. In patients who have CIP, electrophysiological changes can be detected as early as 24 to 48 hours after the onset of ICUAW and often precede clinical findings. Some limitations of EMG are the lack of universal ICU availability and reduced utility for patients who cannot contract their muscles voluntarily (Lacomis, 2023; Plaut & Weiss, 2022). On EMG, both CIP and CIM can have abnormal spontaneous activity at rest. CIP patients typically have reduced recruitment (the successive activation of the same or additional motor units with increasing strength of voluntary muscle contraction) and large prolonged motor units (a single motor nerve and associated muscle fibers that are innervated upon stimulation of the nerve). Patients who have CIM typically have rapid recruitment and shorter motor units. These findings can overlap if a patient has both CIP and CIM (Kramer, 2017; Sandbrink, 2019).
NCS are another part of an EMG that uses electrodes to measure the speed and strength of signals traveling between two or more points. On NCS, compound muscle action potentials (CMAP) are severely reduced in those who have CIM and CIP. In patients who have CIM, the duration of CMAPs are prolonged, which correlates with electrical impulse conduction along the muscle fiber, indicating muscle inexcitability. In those who have CIP, sensory nerve action potentials (SNAP) are reduced (low amplitude). The utility of NCS is limited for patients who have edema or electrical interference (external pacing wires [Kramer, 2017; Lacomis, 2023; Plaut & Weiss, 2022]).
Post-Intensive-Care Syndrome (PICS)
PICS refers to a group of problems patients can experience after surviving critical illnesses and MV (American Thoracic Society, 2020b). PICS is characterized by prolonged impairment in cognition, mental health, and physical functioning that can persist for years following ICU admission. As many as 50% of patients who survive critical illness experience at least one PICS-associated complication, negatively impacting 5-year morbidity and mortality. The physical impairments of PICS arise from patients who experienced ICUAW, with long-term sequelae that can include persistent generalized weakness, poor mobility, falls, joint contractures, and an inability to perform activities of daily living (ADLs). PICS can affect anyone who survives critical illness, even those who were healthy before their illness. People who have existing chronic medical conditions, such as lung disease or muscle disorders, are at higher risk of developing PICS. In addition, people who have psychiatric illness or cognitive impairment (dementia) are more likely to experience more severe symptoms after discharge. Patients receiving MV are at higher risk for developing PICS related both to muscle weakness and to the likelihood of having severe infections, ARDS, low oxygen levels, delirium, and low blood pressure (American Thoracic Society, 2020b; Lacomis, 2023; Mikkelsen et al., 2023a). The term PICS-Family (PICS-F) describes symptoms such as sleep deprivation, anxiety, depression, and grief that close family members experience after a loved one has been in the ICU (Smith & Rahman, 2023).
The reported incidence of PICS and PICS-F varies considerably in the scientific literature based on differences in the study population, length of follow-up, and methods of evaluating impairments. However, PICS and PICS-F are observed across various cultures. The physical impairment component of PICS has been found in 25% to 80% of adult ICU survivors, with the highest prevalence among those who had sepsis. An estimated 80% of adult ICU survivors will develop symptoms consistent with the cognitive dysfunction component of PICS. Although some of these symptoms may improve over time, many patients have persistent effects for years after discharge, particularly patients who had ARDS or sepsis. In addition, as many as 50% of ICU survivors have been diagnosed with post-traumatic stress disorder (PTSD), impacting mental health for years (Smith & Rahman, 2023). In a recent study of 406 adult ICU patients from five US medical centers, Marra and colleagues (2018) evaluated the co-occurrence of PICS impairments at 3- and 12-months post-discharge. They found that 64% and 56% of ICU survivors had one or more PICS impairments after 3 and 12 months, respectively. The co-occurrence of PICS impairments across two or more domains was also found, with cognitive and mental health problems most likely co-occurring over time. PICS-F has been reported in up to 75% of family members of ICU patients, with approximately one-third requiring psychiatric medications to manage symptoms. Family members often experience anxiety and depression, which may exacerbate their physical health problems and contribute to substance use disorder and financial issues (Smith & Rahman, 2023).
Prolonged physical impairments associated with PICS are usually related to the diagnosis of ICUAW during admission. ICUAW can occur due to muscle deconditioning, CIP, or CIM. Muscle deconditioning can result from muscle immobility and disuse, inflammatory mediators, electrolyte imbalances, endocrine dysfunction, vitamin D deficiency, and poor nutritional status. MV compounds these effects by contributing to prolonged bed rest and other forms of immobilization due to the administration of opioids, sedatives, and neuromuscular blockers. These factors result in the weakening of extremity and trunk muscles and the rapid wasting of respiratory musculature (Lacomis, 2023; Smith & Rahman, 2023).
Critically ill patients and those requiring MV experience high levels of physical and psychological stress while admitted to the ICU. These experiences can increase the likelihood of patients who have PICS developing cognitive impairments, including impaired memory, executive functioning, language, attention, and visual-spatial abilities. Impairments in cognitive function may persist for months to years after discharge from the ICU. Among ICU survivors, risk factors for cognitive impairment include pre-existing cognitive deficits, frequent or prolonged periods of hyperglycemia or hypoglycemia, and delirium. There is strong evidence that patients who have new-onset or prolonged delirium while in the ICU are at greater risk for long-term cognitive dysfunction. In addition, patients requiring MV from ARDS were at higher risk for cognitive deficits a year after discharge from the ICU if they experienced extended and profound periods of hypoxia. Currently, the pathophysiology of cognitive dysfunction after critical illness remains unknown (Inoue et al., 2019; Smith & Rayham, 2023).
Depression, anxiety, and PTSD are significant mental health conditions that occur with PICS. These psychiatric sequelae frequently affect critically ill patients and those requiring MV due to ICU experiences that can be isolating, frightening, and dehumanizing. In addition, repetitive exposure to physical pain, discomfort from the illness or ventilator, and disorientation from sedation or delirium can be traumatic experiences for patients in the ICU. Approximately 30% of ICU survivors experience depression, 70% experience anxiety, and up to 50% are diagnosed with PTSD after ICU discharge. A pre-existing history of psychiatric illness, younger age, female gender, lower education level, and alcohol use disorder are all risk factors for the development of mental illness after admission to an ICU. In addition, the administration of sedatives and frequent or recurring episodes of hypoglycemia and hypoxia increase the risk of cognitive dysfunction and depression after ICU admission. HCPs should complete frequent and ongoing comprehensive assessments (physical, cognitive, and psychological) of all patients recovering from critical illness to identify PICS. Because PICS is diagnosed after discharge from the hospital, continuity of care between the ICU, rehabilitation or nursing facilities, and home care is essential (Inoue et al., 2019; Mikkelsen et al., 2023a; Smith & Rahman, 2023).
Treatment for PICS depends upon whether the patient is experiencing physical, cognitive, or mental health impairments. Muscle weakness and deconditioning can be treated with physical therapy (PT), occupational therapy (OT), and exercise programs. For symptoms of depression and anxiety, a combination of medication and therapy services is recommended. A neurocognitive specialist should be considered for patients experiencing difficulty concentrating, thinking, or remembering. Due to the increasing survival rates of patients who have critical illness, many multidisciplinary clinics (physicians, nurses, social workers, pharmacists, and PT and OT professionals) now exist to manage PICS in patients. With early identification of PICS and coordinated multidisciplinary treatment, symptoms can resolve within a few weeks to months after hospitalization. However, some patients will experience prolonged or even permanent impairment related to PICS. Several interventions can reduce the risk of developing PICS during hospitalization, including minimizing sedation and paralytic medications and early weaning from MV. In addition, EM for patients who have critical illness and those requiring MV can reduce the risk of ICUAW and subsequent PICS (American Thoracic Society, 2020b; Mikkelsen, et al., 2023b; Smith & Rahman, 2023).
Preventing Complications Associated with Mechanical Ventilation
Preventative measures implemented immediately upon admission to the ICU can improve mortality rates and clinical outcomes and enhance QOL after discharge. The ABCDEF bundle of care is an evidence-based approach to preventing complications associated with critical illness and MV (Smith & Rahman, 2023). Traditionally, ICU staff have frequently implemented sedation and restraints to facilitate necessary treatments. However, these iatrogenic aspects of care can threaten patient dignity and result in physical, cognitive, and psychological harm. The ICU Liberation Collaborative is a real-world quality improvement (QI) initiative designed to engage health care facilities in the strategic implementation of an ABCDEF bundle utilizing team-based care. Although the ICU Liberation Collaboration has a specific bundle to guide implementation, it is not designed to be a static, rigidly applied protocol. Instead, the bundle can be adapted to institutional preferences and needs. The concepts were designed to assist all ICU programs with varying patient populations (small and large, community and academic, public and governmental). The ICU Liberation Collaboration QI initiative has been implemented in ICUs across the US (Ely, 2018). Numerous studies involving more than 20,000 patients have found that this bundle is associated with a 68% decrease in the likelihood of hospital death within 7 days and a 50% reduction in ICU readmissions. In addition, the ABCDEF bundle is associated with a 25% to 50% reduction in delirium and coma days, a 60% reduction in physical restraints, and a 40% reduction in discharges to long-term care facilities (SCCM, n.d.). The key components of the ABCDEF bundle consist of the following (Ely, 2018; Mart et al., 2019; SCCM, n.d.):
- Assessment, prevention, and management of pain: Pain is a common occurrence in ICUs that is often unrecognized and undertreated. Untreated pain is a risk factor for the development of delirium in ICU patients. HCPs must actively assess and manage pain to prevent complications. Assessment can include self-reporting, observation of behavioral changes, asking the family to help identify pain behaviors, and assuming pain is present. Pain should be assessed frequently in the ICU (every 4 hours) and reassessed (every 1 hours) using validated tools, such as the numerical rating scale (NRS), behavioral pain scale (BPS), or the critical-care pain observation tool (CPOT). The preferred first-line medication is intravenous opioids. Alternative medications (nonsteroidal anti-inflammatory drugs [NSAIDs] and gabapentin) and non-pharmacologic interventions should also be considered to reduce opioid doses. Adequate pain management reduces delirium and facilitates the performance of other components of the ABCDEF bundle (EM and breathing trials).
- Both spontaneous awakening trials (SAT) and spontaneous breathing trials (SBT): Coordinated and protocolized SATs and SBTs are foundational to liberation from MV. SATs involve the cessation of sedatives and narcotics for patients receiving MV. This element focuses on setting a time(s) daily to stop sedation medications, orient the patient to date and time, and conduct an SBT. Daily interruption of sedation can decrease ventilator days, VAP rates, mortality, and other complications, such as ICUAW and PICS. The routine use of SBTs facilitates ventilator weaning and liberation. Protocolized SBTs have reduced ventilator days, length of stay, mortality, and other complications. HCPs must complete a safety screen before initiating SATs or SBTs.
- Choice of analgesia and sedation: HCPs should use goal-directed sedation and analgesia to reduce the overall drug burden and achieve light sedation. This element focuses on safe and effective medication regimens consistent with the pain, agitation, and delirium (PAD) guidelines and the pain, agitation/sedation, delirium, immobility, and sleep (PADIS) guidelines. Routine assessments of pain and sedation levels (at least every 4 hours) should be done using validated measures to prevent over- or under-sedation. The Richmond Agitation-Sedation Scale (RAAS) and the Riker Sedation-Agitation Scale (RAS) are recommended. The goal of sedation and analgesia for critically ill patients should be a calm, alert state, allowing for patient interaction.
- Delirium assessment, prevention, and management: Delirium is a pervasive form of brain failure in critically ill patients that is characterized by waxing and waning confusion. Patients can experience hypoactive (a reduced level of consciousness with fluctuating attention and awareness) or hyperactive (increasing levels of agitation with fluctuating attention and awareness) delirium. It is associated with increased mortality and long-term complications (PICS). HCPs play a critical role in identifying delirium, and screening should occur daily in the ICU. Two of the most common and validated instruments for detecting delirium are the Intensive Care Delirium Screening Checklist (ICDSC) and the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Promoting sleep, reducing noise, and providing EM can help minimize the development of delirium.
- Early mobility and exercise: EM is an essential component of the ABCDEF bundle that is often underutilized. The safety of EM and physical activity in critically ill patients are often a concern for HCPs. Prolonged immobilization can lead to muscle wasting and weakness, PMV, poor functional status, ICUAW, and PICS. EM can decrease delirium, improve functional outcomes, and reduce health care costs. The safety and effectiveness of EM will be discussed in detail below.
- Family engagement and empowerment: Patients who have critical illness and those requiring MV are often unable to communicate with the care team and with their families. Patient-centered care respects the individual patient's preferences and values and informs clinical decision-making. Empowering family members to engage in shared decision-making and facilitating open communication improves patient outcomes. Critical illness can lead to significant psychological problems for patients and family members. HCPs who actively engage with patients and family members can prevent serious complications, including delirium, ICUAW, and PICS.
Evidence for Early Mobilization during Mechanical Ventilation
Although not consistently defined in the literature, the most commonly used definition for EM is any activity beyond a patient's range of motion (ROM) performed within 48 hours of the initiation of MV (Akhtar & Deshmukh, 2021). The American Thoracic Society and the American College of Chest Physicians (CHEST) have set guidelines regarding liberation from MV for critically ill adults, highlighting research about whether critically ill adults who have been on MV for more than 24 hours should be subjected to protocolized rehabilitation directed toward EM. The authors defined rehabilitation as any program targeting mobilization, regardless of who implemented the program (nurse, physical therapist, or another clinician; Girard et al., 2017).
EM and rehabilitation of mechanically vented ICU patients represent a rapidly growing body of literature. Reports about the benefits of mobilizing hospitalized patients have been published since the late 19th century, leading to shorter post-operative bed rest ranging from weeks to just a few hours. In addition, EM of mechanically ventilated patients has been shown to impact short-term and long-term benefits (Hashem, Parker, et al., 2016). Girard and colleagues (2017) concluded in their guidelines for liberation from MV that the desirable consequences of EM outweigh the undesirable consequences. There may be an optimal window to initiate EM based on the rapid onset of muscle loss in the ICU. In addition, the researchers found significant variation in the initiation timing of EM, the duration of mobilization, the types of activities, and the staff who implemented the intervention. EM shortened the duration of MV by 2.7 days, and these patients were more likely to walk at discharge. An analysis estimated that protocolized rehabilitation in the ICU could result in cost savings for each patient. One identified concern for EM was resource allocation. Overall, the authors considered the quality of evidence to be low, based on the study variations described and the small sample sizes (Girard et al., 2017).
Zhang and colleagues (2019) conducted a systematic review to evaluate the impact of EM on critically ill patients in the ICU. The researchers included 23 randomized control trials (RCTs) comprising 2,308 critically ill patients. They found that EM decreased the incidence of ICUAW at discharge by 40%, increased the number of patients who were able to stand (90% vs. 62%), and increased the number of ventilator-free days. Also, EM increased the distance that patients could walk unassisted at hospital discharge (33.4 vs. 0 meters) and boosted the discharge-to-home rate by 16%. In addition, EM improved mortality and adverse event rates, but the differences were not statistically significant. Although the findings support the benefits of EM, the researchers concluded that the quality of evidence was low and that further investigation is needed (Zhang et al., 2019).
In another systematic review, Menges and colleagues (2021) evaluated the effectiveness of systematic EM in improving muscle strength and physical function for mechanically ventilated ICU patients. The researchers reported that the definition of standard of care has not been consistent between studies and may have changed as EM became increasingly implemented in clinical practice. The team hypothesized that the current standard of care might include mobilization approaches that are provided early but not systematically. They identified 12 RCTs comprising 1,304 participants, with two studies comparing early and late mobilization, nine comparing standard care with early EM, and one comparing EM with no mobilization. When weighing standardized EM against late mobilization, the researchers found that patients receiving standardized EM had higher SF-36 Physical Function Domain Scores (PFS) and SF-36 Physical Health Consequence Scores (PCS). These studies also demonstrated a higher proportion of patients reaching walking independence. Weighing studies that evaluated systematic EM against standard EM, the researchers did not find a difference in patients' PFS or PCS scores. Systematic EM improved the time required for independent evaluation. Although EM and standardized EM showed beneficial outcomes, the overall quality of evidence was deemed low based on inconsistent definitions, EM interventions, and small sample sizes (Menges et al., 2021).
More recent studies have shown mixed results on the effectiveness of EM for critically ill patients. For example, the TEAM Study Investigators and the ANZICS Clinical Trials Group (2022) evaluated the effectiveness of an EM intervention compared to usual care for 750 randomly assigned adult patients in the ICU who were undergoing MV. They found that EM intervention did not result in a significantly greater number of days the patients were alive and out of the hospital when compared to the usual care group. It is hypothesized that the variations in outcomes between earlier studies and more recent studies could be explained by the differences in the populations chosen for those studies, the variations in the timing of initiation of EM programs, and the variations in the EM protocols used across the various studies. However, despite these differences in study outcomes, EM programs are still recommended because they have been shown to be safe and cost-effective. More research is needed to determine the effectiveness of EM programs (Rawal & Bakhru, 2023).
Implementation of Early Mobilization for Mechanically Ventilated Patients
Implementing EM for mechanically ventilated patients in the ICU requires a standardized, multidisciplinary approach. HCPs must know the barriers to implementation, safety considerations, mechanisms to assess readiness, and successful implementation approaches. EM is a complex intervention for critically ill patients and those requiring mechanical ventilation. Health care institutions must ensure adequate training and resources for EM programs to succeed (AHRQ, 2017b).
Barriers to Early Mobilization
Evidence has shown that EM can decrease the length of stay and length of time on a ventilator and prevent VAEs, ICUAW, and PICS for mechanically ventilated patients. However, EM is not consistently implemented in ICUs across the US. Many barriers have been discussed in the literature, including ICU culture, HCPs' perspectives on EM, costs of resources, and ICU treatments, such as endotracheal tubes (ETT), sedation, and central line catheters (Hashem, Parker, et al., 2016).
Cultural Barriers
An ICU's culture is a significant and potentially modifiable barrier to EM implementation for mechanically ventilated patients. Critically ill patients require complex and time-consuming care, resulting in the potential for numerous procedures, insufficient coordination, and competing priorities. ICU leadership can overcome these barriers by creating a structured multidisciplinary EM program, emphasizing clear communication, and highlighting the importance of EM. When HCPs create a culture that prioritizes EM, the number of mechanically ventilated patients receiving EM increases three-fold. The Johns Hopkins Hospital medical ICU implemented a structured QI initiative to change the culture and implement EM for all patients. The hospital's ICU leadership changed patient activity and sedation practices and created PT and OT consultation guidelines. The effectiveness of the project was evaluated weekly during multidisciplinary team meetings. This QI initiative decreased the number of ICU days where eligible patients did not receive PT and OT interventions and improved the timing of early PT interventions (Hashem, Parker, et al., 2016).
HCPs' Perspectives on EM
HCPs caring for patients receiving MV may be hesitant to initiate EM due to medical instability. Although EM has been consistently reported to be safe and feasible for critically ill patients, HCPs' knowledge of and training in EM may be inadequate. Akhtar and Deshmukh (2021) conducted a cross-sectional survey study at a tertiary academic institution to evaluate HCPs' knowledge of and attitudes about EM for adult patients who have critical illnesses. The researchers found that 78% of respondents acknowledged the benefits of EM in shortening the length of MV, and 54% believed it maintained muscle strength. In addition, 44% of HCPs considered EM crucial to the care of patients receiving MV but reported that EM should not be initiated until cardiorespiratory status stabilizes. Finally, the respondents identified barriers to EM, including the lack of written guidelines or protocols, limited staff to mobilize patients, inadequate training, excessive sedation, and medical instability (Akhtar & Deshmukh, 2021).
Costs of Resources
As identified by the cross-sectional survey study conducted by Akhtar and Deshmukh (2021), HCPs identified staffing resources needed to implement EM as a barrier in ICUs. The creation and implementation of dedicated mobility teams (nurses and PT professionals) were recommended for EM programs. This multidisciplinary approach would ensure the safe implementation of mobilization for patients receiving MV. These dedicated mobility teams can also provide consistent and timely interventions. Although dedicated mobility teams require additional staffing, studies have shown that these teams did not increase overall costs. By preventing VAEs, decreasing the number of ventilator and ICU days, and reducing the risk of readmission, dedicated mobility teams offer benefits that outweigh additional staffing costs (Hashem, Parker, et al., 2016).
ICU Treatments
Critically ill patients and patients requiring MV often need numerous ICU treatments, including sedation, ETTs, and central line catheters. These treatments are often seen as a barrier to the safe and effective implementation of EM. The widespread use of sedatives or other medications that alter the level of awareness of critically ill patients can impact EM. When EM programs are considered, patient participation in mobility and other physical activities is critical. HCPs should consider sedation minimization when appropriate for each patient by implementing the ABCDEF bundle. As described previously, with this bundle, patients requiring MV undergo daily SATs and SBTs (Hashem, Parker, et al., 2016; Rawal & Bakhru, 2023).
The presence of an ETT is another perceived barrier to EM. Pohlman and colleagues (2010) detailed the steps to initiate PT and OT interventions for 49 patients receiving MV. Patients underwent daily screening for ten contraindications to PT and OT interventions. In addition, they received daily interruption of sedation until wakefulness was achieved. Once achieved, PT and OT interventions were initiated, with a median of only 1.5 days of intubation. HCPs secured the ETT, removed unnecessary noninvasive devices, and disconnected enteral feeding tubes. EM and rehabilitation interventions were completed on 90% of eligible patient days. One-third of the sessions involved moving from a bed to a chair and standing, while 15% involved ambulation. EM interventions were stopped prematurely in only 4% of sessions, usually due to ventilator dyssynchrony (Hashem, Parker, et al., 2016; Pohlman et al., 2010).
The presence of a central line catheter, specifically a femoral catheter, is perceived as a barrier to EM in the ICU. Central line catheters placed in the femoral area have an increased risk of accidental removal, bleeding, or infection compared to other central line locations. Damluji and colleagues (2013) observed 101 patients with a femoral catheter (81% venous, 29% arterial, and 6% hemodialysis) in a medical ICU. These patients completed 253 PT sessions over 210 ICU days, including in-bed exercises (38% of days), supine cycle ergometry (12% of days), and standing or walking (23% of days). There were no catheter-related adverse events.
Safety and Feasibility of EM for Mechanically Ventilated Patients
EM can be associated with safety risks due to illness severity, ETTs, and other invasive treatment modalities used for patients requiring MV. Studies have shown that EM is related to physiologic stress that can alter physiological variables (heart rate [HR], respiratory rate [RR], arterial pressure, and oxygen saturation). Although these are not drastic changes, HR can increase by up to 15 beats per minute, RR can increase by up to 6 breaths per minute, arterial pressure can change by up to 9 mm Hg, and oxygen saturation can drop by over 1 point. The disruptions can significantly impact critically ill patients who already have limited reserves. These physiologic stresses can also produce more severe problems during EM interventions. For patients receiving MV, the most common problem that terminated EM interventions was oxygen desaturations. Other indications for terminating EM interventions included arrhythmias, ventilator asynchrony, patient falls, BP changes, and accidental removal of catheters (Schmidt et al., 2016).
Assessing the readiness of patients who are critically ill or receiving MV for EM should be performed by trained HCPs. Some guidelines recommend applying stringent criteria regarding physiologic parameters, excluding patients from EM and PT activities if they are outside the parameters. However, some researchers argue that these strict criteria will unnecessarily exclude patients from the benefits of EM. Instead, they advocate allowing patients to participate in therapy interventions under the direct care and observation of HCPs, who can monitor BP, HR, and oxygen saturation. In this type of EM program, patients will be assessed individually rather than by strict exclusion criteria (Schmidt et al., 2016). Experts agree that EM should ideally begin within 48 hours of initiation of MV and involve stable ventilator settings, hemodynamic stability, and an ability to follow commands. Although there are risks to implementing mobilization for patients receiving MV, reported rates of adverse events remain low. Per expert consensus, EM and rehabilitation programs are safe and do not pose significant risks as long as they are closely monitored. HCPs can use safety criteria to determine individual patient risk for adverse events related to the initiation of EM programs (Hashem, Parker, et al., 2016; Rawal & Bakhru, 2023).
Strategies for Implementing EM Programs for MV Patients
Effective EM and rehabilitation programs utilize a coordinated, multidisciplinary, team-based approach. The use of the ABCDEF bundle can help HCPs assess patient readiness and minimize barriers to successful EM, including pain, delirium, or oversedation (Ely, 2018). EM programs involve several levels of progressive activity:
- Level 1: Patient lying in bed
- Level 2: Patient sitting on the bed
- Level 3: Patient standing and pivoting
- Level 4: Patient walking (Schmidt et al., 2016)
All four of these levels will require additional caregivers to implement safely and effectively. For example, for level 1 patients, most activities can be completed by a bedside nurse. However, as patients progress to levels 2 through 4, each task will require additional staff. The number of additional staff members will depend upon how many days per week an ICU is implementing EM activities and the number of patients eligible for the program. For example, many programs have reported that six additional staff members (nurses, nursing assistants, PTs, OTs) were needed to successfully implement and sustain an EM program (Schmidt et al., 2016).
Technology to Support EM and Rehabilitation
Several technologies have been used to assist rehabilitation and mobilization activities for patients who have critical illness or those requiring MV. Neuromuscular electrical stimulation (NMES) has been used extensively in PT and rehabilitation practices. NMES delivers low-voltage electrical impulses through electrodes that are placed on the skin of targeted muscle groups. These electrical impulses cause passive contraction of the muscles. There has been increasing interest in using NMES to prevent and treat ICUAW in critically ill patients. Studies have shown that NMES is safe and feasible and can improve muscle strength, mass, and function. These benefits may occur due to cytokine changes similar to the changes observed after active exercise (Hashem, Nelliot, et al., 2016; Hashem, Parker, et al., 2016; Othman et al., 2023).
In-bed cycle ergometry uses a device on which a supine patient can perform passive, active-assisted, or active in-bed cycling. For unconscious patients, passive cycling may limit protein catabolism, preventing complications such as ICUAW. Cycle ergometry does not appear to cause any clinically adverse hemodynamic changes, even within 72 hours of MV initiation. Studies have shown that cycle ergometry significantly increases quadriceps muscle strength, 6-minute walking distance, and QOL scores. In addition, few safety events have been reported among critically ill patients requiring MV. Combining NMES with bedside cycling, known as functional electrical stimulation cycling, could engage patients in active cycling at early stages of critical illness. Functional electrical stimulation cycling can improve short-term and long-term physical and cognitive outcomes in mechanically ventilated patients. A more recent systematic review was conducted to evaluate the effect of in-ICU leg cycle ergometry on clinical outcomes (Takaoka et al., 2020). These researchers found that in-ICU cycling did not result in significant adverse effects but also did not show any differences in the duration of MV, LOS, and physical function compared to those who did not receive the cycling. Additional research into the safety and effectiveness of technology to assist with EM and rehabilitation is warranted (Hashem, Nelliot, et al., 2016; Hashem, Parker, et al., 2016).
Nurse-Driven EM Protocols
The AHRQ (2017b) has prepared a facilitator guide regarding nurse-driven EM protocols. Nurses working in the ICU have the opportunity to lead the implementation of EM programs for patients requiring MV. The goals of nurse-driven EM are to promote a multidisciplinary, team-based focus on instituting EM as part of daily clinical routines to maintain baseline patient mobility and functional levels. Some key features of this nurse-driven protocol include the following:
- Using a multidisciplinary and coordinated approach
- Minimizing sedation use and interrupting sedation daily
- Assessing for and addressing delirium
- Screening patients for eligibility for the highest level of mobility
- Tailoring goals to maximize mobility
- Consulting PT when appropriate (AHRQ, 2017b)
The ABCDEF bundle should guide the implementation of a nurse-driven EM protocol (refer to the specific components above). Multidisciplinary daily rounds should involve an active, real-time discussion of mobility for each patient. HCPs should report on the mobility status of each patient at shift changes and when transferring patients to another unit. The use of signage algorithms can help alert staff to each patient's mobility status and goals. An ICU EM Screening Algorithm and an ICU Early Mobility Protocol by the ARHQ appear in the facilitator guide on the organization's website. HCPs can use the screening algorithm to determine whether a patient is clinically ready for EM (AHRQ, 2017b).
The algorithm starts by reviewing neurological criteria. If a patient successfully meets these criteria, respiratory, circulatory, and other considerations are evaluated. If a patient clears all exclusion criteria, the nurse can initiate the EM protocol with PT. If a patient clears some but not all exclusion criteria, the nurse should discuss the appropriateness of EM with the health care team. The ICU EM Protocol algorithm guides the team through the stages of mobility. The nurse can consult PT and OT if the patient is not currently at their baseline functional status or is not progressing through the algorithm. Health care institutions should consider establishing a mobility technician program to bridge the gap between nurses and PT or OT. Mobility technicians (nursing assistants) can support EM of patients who do not require skilled therapy or more than minimal assistance. Ensuring a multidisciplinary, coordinated, team-based approach to EM can safely optimize outcomes for patients receiving MV (AHRQ, 2017b).
References
Agency for Healthcare Research and Quality. (2017a). Early mobility guide for reducing ventilator-associated events in mechanically ventilated patients. https://www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/hais/tools/mvp/modules/technical/early-mobility-mvpguide.pdf
Agency for Healthcare Research and Quality. (2017b). Nurse-driven early mobility protocols: Facilitator guide. https://www.ahrq.gov/hai/tools/mvp/modules/technical/nurse-early-mobility-protocols-fac-guide.html
Akhtar, P. M., & Deshmukh, P. K. (2021). Knowledge, attitudes, and perceived barriers of healthcare providers toward early mobilization of adult critically ill patients in intensive care unit. Indian Journal of Critical Care Medicine, 25(5), 512-518. https://doi.org/10.5005/jp-journals-10071-23835
American Thoracic Society. (2020a). Mechanical ventilation. https://www.thoracic.org/patients/patient-resources/resources/mechanical-ventilation.pdf
American Thoracic Society. (2020b). What is post intensive care syndrome (PICS)? https://www.thoracic.org/patients/patient-resources/resources/post-intensive-care-syndrome.pdf
Andreae, M. E., & Hong, J. S. (2024). Critical illness myopathy. https://now.aapmr.org/critical-illness-myopathy
Centers for Disease Control and Prevention. (2024a). About chronic diseases. National Center for Chronic Disease Prevention and Health Promotion. https://www.cdc.gov/chronic-disease/about
Centers for Disease Control and Prevention. (2024b). Deaths and mortality. https://www.cdc.gov/nchs/fastats/deaths.htm
Centers for Disease Control and Prevention. (2024c). Living with a chronic condition. https://www.cdc.gov/chronic-disease/living-with/index.html
Centers for Disease Control and Prevention. (2024d). Ventilator-associated pneumonia basics. https://www.cdc.gov/ventilator-associated-pneumonia/about
Cheung, K., Rathbone, A., Melanson, M., Trier, J., Ritsma, B. R., & Allen, M. D. (2021). Pathophysiology and management of critical illness polyneuropathy and myopathy. Journal of Applied Physiology, 130, 1479-1489. https://doi.org/10.1152/japplphysiol.00019.2021
Damluji, A., Zanni, J. M., Mantheiy, E., Colantuoni, E., Kho, M. E., & Needham, D. M. (2013). Safety and feasibility of femoral catheters during physical rehabilitation in the intensive care unit. Journal of Critical Care, 28(4), 535.e9-535.e15. https://doi.org/10.1016/j.jcrc.2013.01.006
Dirkes, S. M., & Kozlowski, C. (2019). Early mobility in the intensive care unit: Evidence, barriers, and future directions. Critical Care Nurse, 39(3), 33-43. https://doi.org/10.4037/ccn2019654
Doiron, K. A., Hoffmann, T. C., & Beller, E. M. (2018). Early intervention (mobilization or active exercise) for critically ill adults in the intensive care unit. Cochrane Database of Systematic Reviews. https://doi.org/10.1002/14651858.CD010754.pub2
Ely, E. W. (2018). The ABCDEF bundle: Science and philosophy of how ICU liberation serves patients and families. Critical Care Medicine, 45(2), 321-330. https://doi.org/10.1097/CCM.0000000000002175
Fadila, M., Rajasurya, V., & Regunath, H. (2022). Ventilator weaning. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK430712
Fan, E., Dowdy, D. W., Colantuoni, E., Mendez-Tellez, P. A., Sevransky, J. E., Shanholtz, C., Himmelfarb, C. R. D., Desai, S. V., Ciesla, N., Herridge, M. S., Pronovost, P., J., & Needham, D. M. (2014). Physical complications in acute lung injury survivors: A two-year longitudinal prospective study. Critical Care Medicine, 42(4), 849-859. https://doi.org/10.1097/CCM.0000000000000040
Girard, T. D., Alhazzani, W., Kress, J. P., Ouellette, D. R., Schmidt, G. A., Truwit, J. D., Burns, S. M., Epstein, S. K., Esteban, A., Fan, E., Ferrer, M., Fraser, G. L., Ng Gong, M. Hough, C. L., Mehta, S., Nanchal, R., Patel, S., Pawlik, A. J., Schweickert, W. D., Sessler, C. N., Strøm, T., Wilson, K. C., & Morris, P. E., on behalf of the ATS/CHEST Ad Hoc Committee on Liberation from Mechanical Ventilation in Adults. (2017). An official American Thoracic Society/American College of Chest Physicians clinical practice guideline: Liberation from mechanical ventilation in critically ill adults. Rehabilitation protocols, ventilator liberation protocols, and cuff leak tests. American Journal of Respiratory and Critical Care Medicine, 195(1), 120-133. https://doi.org/10.1164/rccm.201610-2075ST
Hashem, M. D., Nelliot, A., & Needham, D. M. (2016). Early mobilization and rehabilitation in the ICU: Moving back to the future. Respiratory Care, 61(7), 971-979. https://doi.org/10.4187/respcare.04741
Hashem, M. D., Parker, A. M., & Needham, D. M. (2016). Early mobilization and rehabilitation of patients who are critically ill. Contemporary Reviews in Critical Care Medicine, 150(3), 722-731. https://doi.org/10.1016/j.chest.2016.03.003
Hinkle, J. L., Cheever, K. H., & Overbaugh, K. (2021). Brunner & Suddarth’s textbook of medical-surgical nursing (15th ed.). Wolters Kluwer.
Huang, H.-Y., Huang, C.-Y., & Li, L.-F. (2022). Prolonged mechanical ventilation: Outcomes and management. Journal of Clinical Medicine, 11(9), 2451. https://doi.org/10.3390%2Fjcm11092451
Hyzy, R. C., & McSparron, J. I. (2024). Overview of initiating invasive mechanical ventilation in adults in the intensive care unit. UpToDate. Retrieved July 5, 2024, from https://www.uptodate.com/contents/overview-of-initiating-invasive-mechanical-ventilation-in-adults-in-the-intensive-care-unit
Inoue, S., Hatakeyama, J., Kondo, Y., Hifuni, T., Sakuramoto, H., Kawasaki, T., Taito, S., Nakamura, K., Unoki, T., Kawai, Y., Kenmotsu, Y., Saito, M., Yamakawa, K., & Nishida, O. (2019). Post-intensive care syndrome: Its pathophysiology, prevention, and future directions. Acute Medicine & Surgery, 6(3), 233-246. https://doi.org/10.1002/ams2.415
Kho, M. E., & Connolly, B. (2023). From strict bedrest to early mobilization. Critical Care Clinics, 39(3), 479-502. https://doi.org/10.1016/j.ccc.2023.01.003
King Han, M., & Mirza, S. H. (2023). Management and prognosis of patients requiring prolonged mechanical ventilation. UpToDate. Retrieved July 5, 2024, from https://www.uptodate.com/contents/management-and-prognosis-of-patients-requiring-prolonged-mechanical-ventilation
Klompas, M. (2019). Ventilator-associated events: What they are and what they are not. Respiratory Care, 64(8), 953-961. https://doi.org/10.4187/respcare.07059
Klompas, M. (2023). Epidemiology, pathogenesis, microbiology, and diagnosis of hospital-acquired and ventilator-associated pneumonia in adults. UpToDate. Retrieved July 10, 2024, from https://www.uptodate.com/contents/epidemiology-pathogenesis-microbiology-and-diagnosis-of-hospital-acquired-and-ventilator-associated-pneumonia-in-adults
Kollef, M. H. (2023). Clinical presentation and diagnostic evaluation of ventilator-associated pneumonia. UpToDate. Retrieved July 10, 2024, from https://www.uptodate.com/contents/clinical-presentation-and-diagnostic-evaluation-of-ventilator-associated-pneumonia
Kramer, C. L. (2017). Intensive care unit-acquired weakness. Neurologic Clinics, 35(4), 723-736. https://doi.org/10.1016/j.ncl.2017.06.008
Lacomis, D. (2023). Neuromuscular weakness related to critical illness. UpToDate. Retrieved July 12, 2024, from https://www.uptodate.com/contents/neuromuscular-weakness-related-to-critical-illness
Lutz, E. (2020). Biology of ventilation [Image]. https://commons.wikimedia.org/wiki/File:Biology_of_ventilation.gif
Marra, A., Pandharipande, P. P., Girard, T. D., Patel, M. B., Hughes, C. G., Jackson, J. C., Thompson, J. L., Chandrasekhar, R., Wesley Ely, E., & Brummel, N. E. (2018). Co-occurrence of post-intensive care syndrome problem among 406 survivors of critical illness. Critical Care Medicine, 46(9), 1393-1401. https://doi.org/10.1097/CCM.0000000000003218
Mart, M. F., Brummel, N. E., & Ely, E. W. (2019). The ABCDEF bundle for the respiratory therapist. Respiratory Care, 64(12), 1561-1573. https://doi.org/10.4187/respcare.07235
Menges, D., Seiler, B., Tomonaga, Y., Schwenkglenks, M., Puhan, M. A., & Yebyo, H. G. (2021). Systematic early versus late mobilization or standard early mobilization in mechanically ventilated adult ICU patients: Systematic review and meta-analysis. Critical Care, 25(1), 16. https://doi.org/10.1186/s13054-020-03446-9
Mikkelsen, M. E., Netzer, G., & Iwashyna, T. (2023a). Post-intensive care syndrome (PICS) in adults: Clinical features and diagnostic evaluation. UpToDate. Retrieved July 16, 2024, from https://www.uptodate.com/contents/post-intensive-care-syndrome-pics-in-adults-clinical-features-and-diagnostic-evaluation
Mikkelsen, M. E., Netzer, G., & Iwashyna, T. (2023b). Post-intensive care syndrome (PICS): Treatment and prognosis. UpToDate. Retrieved July 16, 2024, from https://www.uptodate.com/contents/post-intensive-care-syndrome-pics-treatment-and-prognosis
Mora Carpio, A. L., & Mora, J. I. (2023). Ventilator management. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK448186
National Healthcare Safety Network. (2024). Ventilator-associated event (VAE). Centers for Disease Control and Prevention. https://www.cdc.gov/nhsn/pdfs/pscmanual/10-vae_final.pdf
Needham, D. M., Wozniak, A. W., Hough, C. L., Morris, P. E., Dinglas, V. D., Jackson, J. C., Mendez-Tellez, P. A., Shanholtz, C., Ely, E. W., Colantuoni, E., & Hopkins, R. O. (2014). Risk factors for physical impairment after acute lung injury in a national, multicenter study. American Journal of Respiratory and Critical Care Medicine, 189(10), 1214-1224. https://doi.org/10.1164/rccm.201401-0158OC
Othman, S. Y., Elbiaa, M. A., Mansour, E. R., El-Menshawy, A. M., & Elsayed, S. M. (2023). Effect of neuromuscular electrical stimulation and early physical activity on ICU-acquired weakness in mechanically ventilated patients: A randomized controlled trial. Nursing in Critical Care, 29(3), 584-596. https://doi.org/10.1111/nicc.13010
Patel, B. K. (2024). Overview of mechanical ventilation. https://www.merckmanuals.com/professional/critical-care-medicine/respiratory-failure-and-mechanical-ventilation/overview-of-mechanical-ventilation
Physiopedia. (n.d.-a). Early mobility assessment for critically ill patients. Retrieved July 5, 2024, from https://www.physio-pedia.com/Early_Mobility_Assessment_for_Critically_Ill_Patients
Physiopedia. (n.d.-b). ICU acquired weakness. Retrieved July 13, 2024, from https://www.physio-pedia.com/ICU_Acquired_Weakness
Plaut, T., & Weiss, L. (2022). Electrodiagnostic evaluation of critical illness neuropathy. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK562270
Pohlman, M. C., Schweickert, W. D., Pohlman, A. S., Nigos, C., Pawlik, A. J., Esbrook, C L., Spears, L., Miller, M., Franczyk, M., Deprizio, D., Schmidt, G. A., & Bowman, A. (2010). Feasibility of physical and occupational therapy beginning from initiation of mechanical ventilation. Critical Care Medicine, 38(11), 2089-2094. https://doi.org/10.1097/CCM.0b013e3181f270c3
Rawal, H., & Bakhru, R. N. (2023). Early mobilization in the ICU. Chest: Critical Care, 2(1), 100038. https://doi.org/10.1016/j.chstcc.2023.100038
Sandbrink, F. (2019). Motor unit recruitment in EMG. https://emedicine.medscape.com/article/1141359-overview
Schmidt, U. H., Knecht, L., & MacIntyre, N. R. (2016). Should early mobilization be routine in mechanically ventilated patients? Respiratory Care, 61(6), 867-875. https://doi.org/10.4187/respcare.04566
Smith, S., & Rahman, O. (2023). Post intensive care syndrome. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK558964
Society of Critical Care Medicine. (n.d.). ICU liberation bundle (A-F). Retrieved July 18, 2024, from https://www.sccm.org/iculiberation/abcdef-bundles
Society of Critical Care Medicine. (2024). Critical care statistics. https://www.sccm.org/Communications/Critical-Care-Statistics
Takaoka, A., Utgikar, R., Rochwerg, B., Cook, D. J., & Kho, M. E. (2020). The efficacy and safety of in-intensive care unit leg-cycle ergometry in critically ill adults: A systematic review and meta-analysis. Annals of the American Thoracic Society, 17(10), 1289-1307. https://doi.org/10.1513/AnnalsATS.202001-059OC
Taylor, C. (2021). Intensive care unit-acquired weakness. Anaesthesia and Intensive Care Medicine, 22(2), 81-84. https://doi.org/10.1016/j.mpaic.2020.12.006
The TEAM Study Investigators and the ANZICS Clinical Trials Group. (2022). Early active mobilization during mechanical ventilation in the ICU. The New England Journal of Medicine, 387(19), 1747-1758. https://doi.org/10.1056/NEJMoa2209083
Vanhorebeek, I., Latronico, N., & Van den Berghe, G. (2020). ICU-acquired weakness. Intensive Care Medicine, 46(4), 637-653. https://doi.org/10.1007%2Fs00134-020-05944-4
Yang, T., Li, Z., Jiang, L., & Xi, X. (2018). Corticosteroid use and intensive care unit-acquired weakness: A systematic review and meta-analysis. Critical Care, 22(187), 2018. https://doi.org/10.1186/s13054-018-2111-0
Z'Graggen, W. J., & Tankisi, H. (2020). Critical illness myopathy. Journal of Clinical Neurophysiology, 37(3), 200-204. https://doi.org/10.1097/WNP.0000000000000652
Zhang, L., Hu, W., Cai, Z., Liu, J., Wu, J., Deng, Y., Yu, K., Chen, X., Zhu, L., Ma, J., & Qin, Y. (2019). Early mobilization of critically ill patients in the intensive care unit: A systematic review and meta-analysis. PLoS One, 14(10), e0223185. https://doi.org/10.1371/journal.pone.0223185