About this course:
This 4-hour course reviews the indications for ostomy placement, including the pathophysiology of various underlying conditions that may require an ostomy. In addition, this course outlines the various ostomy-related surgeries and ostomy-related complications. Finally, this course will address postoperative ostomy management and care, including patient education and discharge planning.
Course preview
This 4-hour course reviews the indications for ostomy placement, including the pathophysiology of various underlying conditions that may require an ostomy. In addition, this course outlines the various ostomy-related surgeries and ostomy-related complications. Finally, this course will address postoperative ostomy management and care, including patient education and discharge planning.
After this activity, learners will be prepared to:
- Review the anatomy and physiology of the gastrointestinal (GI) system
- Discuss the indications for ostomy placement and the pathophysiology of various underlying conditions that may require an ostomy
- Describe various ostomy-related surgeries
- Discuss various ostomy-related complications, including prevention and treatment strategies
- Describe the principles of routine ostomy management and care
- Discuss postoperative ostomy-related patient education for discharge planning
Healthcare providers (HCPs) across various clinical settings will care for people with newly placed or existing ostomies. An ostomy (i.e., colostomy, ileostomy, or urostomy) is a surgically created opening in the abdominal wall that allows for the external diversion of urine or stool. The surgical procedure results in a stoma (the end portion of the small or large intestine) that protrudes through the abdominal wall. An estimated 1.3 million people worldwide live with an ostomy, with approximately 750,000 of these people living in North America (US and Canada; Registered Nurses' Association of Ontario [RNAO], 2019). Approximately 100,000 new colostomy or ileostomy surgeries are performed in the US each year (Hendren et al., 2015).
The creation of an ostomy may be permanent or temporary based on the underlying etiology, which can be diverticulitis, colorectal cancer, bowel obstruction, or inflammatory bowel disease (IBD; i.e., Crohn's disease or ulcerative colitis [UC]). A newly created ostomy is a life-changing event, impacting the person's physical and psychological well-being and quality of life (QOL). Approximately 20% to 70% of people with ostomies experience stoma-related complications (i.e., ostomy leakage). In addition, one-third of people with a colostomy and two-thirds with a urostomy or an ileostomy experience peristomal skin problems. Living with an ostomy can also negatively impact a person’s psychological health and social functioning (e.g., body image, sexual function, mood, and daily activities). Financially, ostomy supplies and ostomy-related complications can be expensive. Comprehensive ostomy care requires HCPs to be knowledgeable about the various types of ostomies, indications for placement, and physical and psychological care required for people with ostomies (RNAO, 2015).
Anatomy and Physiology of the GI System
The digestive system comprises the GI tract and the liver, pancreas, and gallbladder (i.e., solid organs). The GI tract consists of hollow organs (i.e., the mouth, esophagus, stomach, small intestine, large intestine, and anus). The hollow organs are joined in a long, twisting tube from the mouth to the anus. Abnormalities in the GI tract are numerous and represent various pathologies, including bleeding, perforation, obstruction, infectious, and malignancy. Like other organ systems, the GI tract is subject to circulatory disturbances, disruptions of nervous system control, and aging. In addition, many extrinsic factors can interfere with normal GI function. For example, stress and anxiety can lead to indigestion, anorexia, constipation, or diarrhea. In addition, inadequate or abrupt changes in dietary intake can significantly impact the GI tract. Therefore, when people present with GI symptoms, HCPs should assess for physical and mental contributing factors (Hinkle & Cheever, 2018; NIDDK, 2017d).
Anatomy of the GI System
The GI tract is up to 30 feet long in adults. The esophagus is a hollow muscular tube that is approximately 10 inches long. It is located in the mediastinum, anterior to the spine and posterior to the trachea and heart. The esophagus passes through the diaphragmatic hiatus, an opening in the diaphragm. The remaining portion of the GI tract is located in the peritoneal cavity. The stomach is a hollow muscular organ that stores food, secretes digestive fluids, and propels partially digested food (i.e., chyme) into the small intestine. It is located in the left upper portion of the abdomen under the left lobe of the liver and the diaphragm. The small intestine is the longest section of the GI tract, accounting for two-thirds (20-25 feet) of the total length. The small intestine has three parts that fold back and forth on themselves: the duodenum, jejunum, and the ileum. The ileum terminates at the ileocecal valve, which controls the flow of digested material from the ileum into the cecal portion of the large intestine. This valve also prevents the reflux of bacteria into the small intestine (Hinkle & Cheever, 2018; NIDDK, 2017d).
The large intestine consists of an ascending segment on the right side of the abdomen, a transverse segment that extends from right to left in the upper abdomen, and a descending segment on the left side of the abdomen. The terminal portion of the large intestine includes the sigmoid colon, rectum, and anus. The internal and external anal sphincters regulate the anal outlet. The GI tract receives blood from the thoracic and abdominal aorta arteries. Oxygen and nutrients are supplied to the stomach (via the gastric artery) and small intestine (via the superior and inferior mesenteric arteries). Blood flow to the GI tract accounts for 20% of the total cardiac output, increasing significantly after eating. Five large veins—the superior mesenteric, inferior mesenteric, gastric, splenic, and cystic—make up the portal venous system. This system transports blood to the liver, where it is distributed throughout and collected into the hepatic veins, eventually terminating in the inferior vena cava. The GI tract is regulated by sympathetic and parasympathetic portions of the autonomic nervous system (ANS). The sympathetic nerves decrease gastric secretions and motility and constrict the sphincters and blood vessels. In contrast, parasympathetic nerves stimulate peristalsis and increase secretory activities (Hinkle & Cheever, 2018; NIDDK, 2017d). See Figure 1 for components of the digestive system.
Figure 1
Components of the Digestive System
Physiology of the GI System
The digestive system plays an important role in providing nutrients to the cells in the body for energy, growth, and repair. These nutrients are derived from the intake of foods that contain proteins, fats, carbohydrates, vitamins, and minerals. The primary functions of the GI tract include digestion, absorption, and elimination. First, digestion breaks food particles into their molecular form; proteins are broken down into amino acids, fats into fatty acids and glycerol, and carbohydrates into simple sugars. Next, these small nutrient molecules are absorbed into the bloodstream. Finally, undigested, unabsorbed, and other waste products are eliminated. Various secretions within the GI tract aid in the digestion, absorption, and elimination of food and nutrients. The digestion process begins with chewing (i.e., mechanical breakdown of food into small particles) and swallowing (i.e., chemical breakdown by digestive enzymes). Swallowing is a voluntary act regulated by the medulla oblongata in the central nervous system (CNS). Next, the food is propelled into the upper esophagus, where esophageal peristalsis (i.e., rhythmic contractions of the smooth muscles of the esophagus) continues to push the food bolus toward the stomach. Th
...purchase below to continue the course
After food enters the stomach, the stomach muscles mix the food. Highly acidic fluid is secreted to break food into absorbable components (chyme) and aid in destroying ingested bacteria. Hormones, neuroregulators, and local regulators found in gastric secretions control gastric motility and secretion production. Peristaltic contractions in the stomach propel the chyme toward the pylorus. Food remains in the stomach for 30 minutes to several hours, depending on the volume, osmotic pressure, and chemical composition of the gastric contents. Similarly, the pyloric sphincter controls the rate at which chyme enters the small intestine to allow for efficient absorption (Hinkle & Cheever, 2018; NIDDK, 2017d).
As the chyme enters the duodenum of the small intestine, enzymes (i.e., amylase, lipase, and bile) are excreted from the pancreas, liver, gallbladder, and glands in the intestinal wall. Pancreatic enzymes have an alkaline pH that neutralizes the acid entering from the stomach; this includes trypsin (which aids in the digestion of protein), amylase (which aids in the digestion of starch), and lipase (which aids in the digestion of fats). These secretions travel from the pancreatic duct, emptying into the common bile duct. Bile (which aids in emulsifying ingested fats) is stored in the gallbladder and secreted by the liver. The sphincter of Oddi controls the flow of bile, while hormones, neuroregulators, and local regulators in the intestinal secretions control GI motility and secretion production. Two types of contractions occur regularly in the small intestine in response to chyme: segmentation contractions and intestinal peristalsis. Segmentation contractions produce a churning motion to move intestinal contents back and forth, whereas intestinal peristalsis propels the contents toward the colon. Chyme stays in the small intestine for 3 to 6 hours to allow for the continual breakdown of proteins, fats, and carbohydrates and the absorption of nutrients. Small, fingerlike projections called villi line the entire intestine and allow for the absorption of nutrients. Nutrients are absorbed in specific locations along the small intestine and duodenum, and proteins, fats, carbohydrates, sodium, and chloride are absorbed in the jejunum. Finally, vitamin B12 and bile salts are absorbed in the ileum (Fish & Burns, 2021; Hinkle & Cheever, 2018).
As the residual intestinal waste reaches the terminal ileum (typically within 4 hours of eating), it slowly passes into the right colon through the ileocecal valve. The valve opens with each peristaltic wave of the small intestine, allowing some contents to move into the colon. Bacteria within the large intestine assist in the breakdown of undigested and unabsorbed proteins and bile salts. In addition, two colonic secretions (i.e., an electrolyte solution and mucus) are added to the residual material. The electrolyte solution, which is primarily bicarbonate, neutralizes the end products formed by the colonic bacterial action. The mucus provides adherence for the fecal mass and protects the colonic mucosa from the intestinal contents. Intestinal contents move slowly through the colon, aided by a weak peristaltic action. This slow movement allows for the efficient reabsorption of water and electrolytes. Occasionally, stronger, Intermittent peristaltic waves propel the contents for considerable distances. This usually occurs when intestine-stimulating hormones are released or when another meal is eaten. Finally, the waste material reaches and distends the rectum. This process usually takes 12 hours, but as much as one-fourth of the waste material from a meal may be in the rectum after three days (Azzouz & Sharma, 2021; Hinkle & Cheever, 2018).
Fecal matter is about 75% fluid and 25% solid material, including undigested foods, inorganic materials, water, and bacteria. A large portion of the fecal material is non-dietary in origin (i.e., secretions of the GI tract), making the composition relatively unaffected by alterations in the diet. The characteristic brown color is produced from the breakdown of bile by the intestinal bacteria. Similarly, chemicals formed by intestinal bacteria create the odor associated with feces. The GI tract contains approximately 150 mL of gasses (i.e., methane, hydrogen, sulfide, and ammonia) that are absorbed into the portal circulation and detoxified by the liver or expelled from the rectum as flatus. Defecation of stool begins with the distention of the rectum. This distention initiates reflex contractions of the rectal musculature and relaxes the internal anal sphincter. The internal anal sphincter is controlled by the ANS and is usually closed. In contrast, the external sphincter is under the conscious control of the cerebral cortex. Therefore, when defecation occurs, the external anal sphincter voluntarily relaxes, allowing colonic contents to be expelled (Hinkle & Cheever, 2018; NIDDK, 2017d).
Indications for Ostomy Placement
According to the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK, 2014), 60 to 70 million people in the US are affected by digestive diseases, including chronic constipation, diverticular disease, gastroesophageal reflux disease (GERD), gastrointestinal infections, IBD, irritable bowel syndrome (IBS), liver disease, and pancreatitis. GI diseases account for more than 48.3 million medical office visits and approximately 21.7 million hospital admissions annually. In addition, these diseases lead to approximately 246,000 deaths and cost more than $141.8 billion annually. The creation of an ostomy is a surgical treatment modality for some digestive diseases or complications related to those diseases. For example, an ostomy may be placed to decompress the colon (i.e., due to bowel obstruction) or to create a fecal diversion as a treatment modality for pathological GI diseases, including congenital anomalies, diverticulitis, bowel obstruction, IBD, traumatic disruption of the intestinal tract, or GI malignancy such as colorectal cancer (Francone, 2021).
Diverticular Disease
A diverticulum is a saclike herniation of the mucosal and submucosal layers of the colon, extending through a defect in the muscle layer (see Figure 2). High intraluminal pressure, low volume in the colon (i.e., fiber-deficient contents), and decreased muscle strength in the colon wall (i.e., when hardened fecal masses lead to muscular hypertrophy) all contribute to the formation of a diverticulum. Diverticula most commonly occur in the colon but can occur anywhere in the GI tract. Diverticulosis occurs when multiple diverticula are present without inflammation or symptoms. Diverticular disease is common, affecting nearly half of Americans over 65. In addition, the prevalence of diverticular disease increases with age, affecting nearly all adults over the age of 90. Although diverticulosis is common, 80% of people will never develop symptoms or complications related to the disease. For 20% of people with diverticulosis, mild symptoms can include bowel irregularity with intervals of alternating constipation and diarrhea, nausea, anorexia, and bloating or abdominal distention. A low-fiber diet, obesity, smoking history, regular use of non-steroidal anti-inflammatory drugs (NSAIDs) or acetaminophen (Tylenol), and family history are all risk factors for diverticular disease (Hinkle & Cheever, 2018; NIDDK, 2021a, 2021c).
Diverticulitis occurs when a diverticulum becomes inflamed, usually due to bowel contents accumulating in the diverticular sac (see Figure 2). Over time, the accumulated bowel contents decompose, leading to inflammation and infection. Inflammation can also occur if the diverticulum becomes obstructed. The combination of inflammation and the weakened colonic wall of the diverticulum can result in perforation or other complications, including an abscess, fistula formation, obstruction, peritonitis, and hemorrhage. When diverticulitis occurs, 70% of people will report an acute onset of mild to severe left lower quadrant pain. In addition to abdominal pain, people may report a change in bowel habits (usually constipation), nausea, fever, and leukocytosis. If an abscess develops, an abdominal mass may also be palpable. In addition, inflamed diverticula can erode areas adjacent to arterial branches, causing significant rectal bleeding. Recurrent episodes of diverticulitis may cause chronic complications, including colovesicular fistula (i.e., a connection or tunnel between the colon and bladder) and colovaginal fistula (i.e., between the colon and vagina) formation. Repeated inflammation can lead to scar tissue and fibrotic strictures that narrow the colon and increase the risk of intestinal obstruction. Although acute diverticulitis usually resolves with medical management, surgical intervention (i.e., primary resection with anastomosis or creation of an ostomy) may be needed if perforation, an abscess, peritonitis, or obstruction occur (Hinkle & Cheever, 2018; NIDDK, 2021a, 2021c).
Figure 2
Diverticulosis and Diverticulitis
Intestinal Obstruction
An intestinal obstruction occurs when a blockage prevents the normal flow of intestinal contents through the intestinal tract. Mechanical obstruction occurs due to pressure in the intestinal wall from a tumor, stricture, adhesion, intussusception, hernia, or abscess. Functional or paralytic obstruction occurs when the intestinal musculature cannot propel the contents through the bowel (i.e., due to muscular dystrophy, amyloidosis, a neurological disorder such as Parkinson's disease, or an endocrine disorder such as diabetes). Intestinal obstructions can be partial or complete. The severity depends on (a) the region of bowel affected and (b) the degree to which the lumen is occluded or the vascular supply is disrupted. Although bowel obstructions can occur in the large intestine, they are more common in the small intestine. Adhesions are the primary cause of small bowel obstructions, followed by tumors, Crohn's disease, hernias, and intussusception (Hinkle & Cheever, 2018; Mayo Clinic, 2021).
When a bowel obstruction occurs, intestinal contents, fluids, and gas accumulate proximal to the obstruction. Abdominal distention and fluid retention cause a decrease in the absorption of fluids and the stimulation of more gastric secretions. As the abdominal distention increases, the pressure in the intestinal lumen increases, causing a decrease in arteriolar and venous capillary pressure. This change in pressure leads to edema, necrosis, and perforation of the intestinal wall. Initial symptoms of a small bowel obstruction include abdominal discomfort that is crampy and wavelike due to persistent peristalsis above and below the obstruction. The person may pass blood or mucus through the rectum but no fecal matter or flatus. If the intestinal obstruction is complete, peristaltic waves will become vigorous and eventually reverse direction, propelling intestinal contents towards the mouth. When vomiting occurs, the person will lose hydrogen ions and potassium from the stomach, subsequently leading to hypochloremia and hypokalemia. If persistent fluid losses occur, the person can develop dehydration and hypovolemia. Many partial small bowel obstructions can be medically managed by decompressing the bowel through a nasogastric (NG) tube. Surgical management is necessary for a complete small bowel obstruction to reduce the risk of intestinal strangulation and tissue necrosis (Hinkle & Cheever, 2018; Mayo Clinic, 2021).
Large bowel obstructions usually occur in the sigmoid colon secondary to tumors, diverticulitis, IBD, or fecal impaction. The colon can distend considerably beyond its normal capacity, so symptoms develop and progress slowly with large bowel obstructions. Constipation may be the only symptom for weeks until the abdomen becomes markedly distended. Dehydration is less likely to occur with a large bowel obstruction because the colon can absorb fluid contents. Many large bowel obstructions can be medically managed with NG aspiration and decompression. However, surgical management with a temporary or permanent colostomy may be necessary (Hinkle & Cheever, 2018; Mayo Clinic, 2021).
IBD
IBD refers to a group of chronic GI diseases (Crohn's disease and UC) resulting in inflammation, ulceration, or both within the bowel. Approximately 1 to 1.3 million Americans have IBD. Risk factors for IBD include family history (particularly a first-degree relative), being Caucasian or of Ashkenazi Jewish background, and living in a northern climate or urban area. Women, current smokers, and people between the ages of 20 and 29 are at the greatest risk for Crohn's disease. Men, ex-smokers and nonsmokers, people between the ages of 15 and 30, and those older than 60 are at greater risk for UC. Although the exact cause of IBD is unknown, environmental triggers (e.g., exposure to air pollutants), food, tobacco, and viral illness can trigger a cell-mediated immune response and inflammation in people with a genetic predisposition (Hinkle & Cheever, 2018; NIDDK, 2017a, 2020a).
Crohn's Disease
Crohn's disease is a chronic condition with alternating periods of exacerbation and remission that cause inflammation and irritation in the GI tract. The inflammation can target anywhere in the GI tract but most commonly occurs in the distal ileum and the ascending colon, with 35% of affected people with ileitis (only ileal involvement), 45% with ileocolitis (involvement of the ileum and colon), and 20% with granulomatous colitis (only colon involvement; see Figure 3). The prevalence of Crohn's disease continues to increase, affecting more than 500,000 people in the US. Crohn's disease begins with inflammation and abscesses in the intestinal glands (i.e., crypts) that develop into small, focal ulcers. Over time, these lesions deepen into longitudinal and transverse ulcers that are separated by edematous patches, creating the characteristic cobblestone appearance of the affected bowel. As inflammation extends into the peritoneum, fistulas and fissures can form. In addition, granulomas (i.e., a lump or nodule made from a clump of immune cells or white blood cells) can develop in the lymph nodes, in the peritoneum, and through the layers of the bowel. As Crohn's disease progresses, the bowel wall thickens and becomes fibrotic, while the intestinal lumen narrows. Adhesions can occur when diseased bowel loops adhere to the normal bowel loops surrounding them (Hinkle & Cheever, 2018; NIDDK, 2017a).
Scar tissue and granulomas interfere with the ability of the intestine to transport products through the constricted bowel lumen, leading to crampy abdominal pain (usually in the right lower quadrant) and diarrhea. Abdominal pain worsens after eating due to increased peristaltic activity. As a result, people with Crohn's disease often avoid eating, leading to weight loss, malnutrition, and secondary anemia. Ulcers in the membranous lining of the intestine and inflammatory changes cause the swollen intestine to empty irritating discharge into the colon. In addition, Crohn's disease disrupts absorption, leading to chronic diarrhea and nutritional and fluid deficits. Other chronic symptoms can include anorexia and steatorrhea (i.e., excess fat in the feces). The medical management of Crohn's disease aims to reduce inflammation, suppress inappropriate immune responses, and allow bowel rest to promote healing. Nutritional therapy can include oral hydration and a low-residue, high-protein, high-calorie diet to reduce inflammation and control pain and diarrhea. Pharmacologic therapy is also used to manage diarrhea, abdominal pain, and inflammation. Between 60% and 70% of people with Crohn's disease require surgery for recurrent partial intestinal obstructions, complete intestinal obstructions, intractable fistulas, or intractable abscesses (Hinkle & Cheever, 2018; NIDDK, 2017b, 2017c).
Figure 3
Crohn's Disease Versus UC

UC
An estimated 600,00 to 900,000 people in the US have UC. UC is a chronic inflammatory and ulcerative disease of the mucosal and submucosal layers of the colon and rectum. The inflammatory changes typically begin in the rectum and progress proximally through the colon (see Figure 3). Like Crohn's disease, UC is characterized by unpredictable periods of exacerbation and remission. Symptoms can range from mild to severe and begin suddenly or start gradually and worsen over time. Within the superficial mucosa of the colon, UC causes numerous ulcerations, diffuse inflammation, and shedding of the colonic epithelium. The mucosa becomes edematous and inflamed, and the ulcerations can cause bleeding. As UC progresses, the bowel narrows, shortens, and thickens due to muscular hypertrophy and fat deposits. Unlike Crohn's disease, the inflammatory changes of UC do not occur through the entire wall of the colon; therefore, fistulas, obstructions, abscesses, and fissures are not common. UC increases the chance of getting colorectal cancer. This risk increases further when UC affects more of the large intestine, is more severe, starts at a younger age, or has been present for longer (Hinkle & Cheever, 2018; NIDDK, 2020a).
Symptoms of UC usually include bouts of abdominal pain (usually in the left lower quadrant) and diarrhea, with the passage of mucus, pus, and blood. People with UC may also report intermittent tenesmus, which is cramping rectal pain with a feeling of incomplete defecation. Depending on the number of ulcerations present, bleeding can range from mild to severe, resulting in fatigue and anemia. Based on the severity of symptoms, UC can be classified as mild (fewer than four bowel movements per day with occasional bleeding), severe (more than six bowel movements per day with blood most of the time), or fulminant (severe and sudden onset of more than 10 bloody bowel movements per day). As UC worsens, people can experience anorexia, weight loss, fevers, vomiting, electrolyte imbalances, and dehydration. Similar to Crohn's disease, the medical management of UC aims to reduce inflammation, suppress inappropriate immune responses, and allow bowel rest to promote healing. Nutritional and pharmacological therapies are similar to those for Crohn's disease (see above). Approximately one-third of people with severe UC will require surgical intervention for the presence of colon cancer or colonic dysplasia/polyps, megacolon (abnormal dilation of the colon), severe and intractable bleeding, or perforation. UC can be cured with surgical ostomy placement, while Crohn's cannot be cured by surgical ostomy placement because lesions can develop anywhere throughout the GI tract (Hinkle & Cheever, 2018; NIDDK, 2020b, 2020c).
Colorectal Cancer
The colon and rectum are the third most common site for new cancer in the US. Approximately 145,000 new cases and 50,000 deaths from colorectal cancer occur annually in this country. In addition, colorectal cancer is the third leading cause of cancer death for both men and women and the second leading cause of cancer death among all adults. The most significant risk factor for colorectal cancer is older age, with diagnosis most frequently occurring between 65 and 74. However, recent trends show an increasing incidence of colorectal cancer in adults under 50. The exact cause of colorectal cancer is unknown, but numerous risk factors have been identified. Approximately 20% of people with colorectal cancer have a family history (American Cancer Society, 2020a; Hinkle & Cheever, 2018; National Cancer Institute [NCI], 2021). Table 1 outlines many of the risk factors for colorectal cancer.
Table 1
Risk Factors for Colorectal Cancer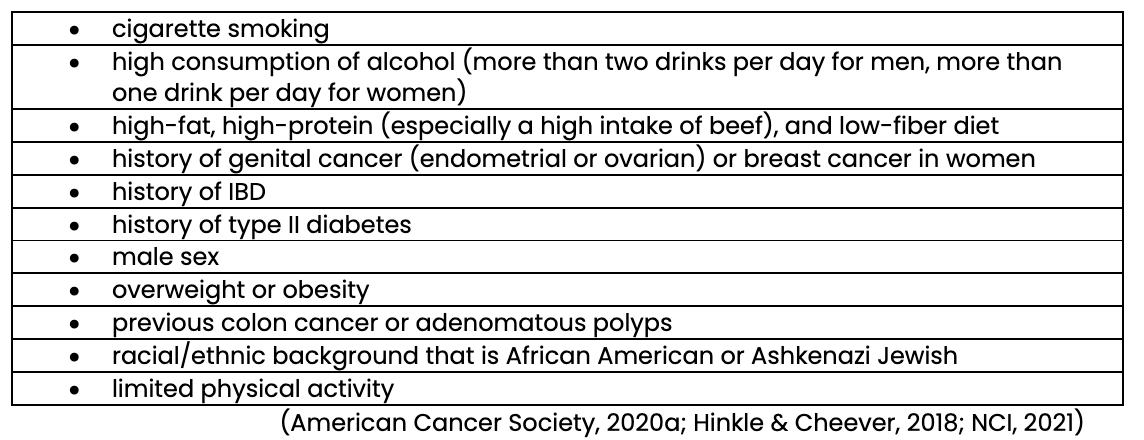
Prevention and early screening are critical to detect and reduce mortality rates for colorectal cancer. When detected in the early stages (i.e., before it spreads), this cancer’s 5-year survival rate is 90%; however, only 39% of cases are detected at an early stage (Hinkle & Cheever, 2018; NCI, 2021).
Colorectal cancer usually starts as a benign polyp that becomes malignant. Adenocarcinoma, arising in the epithelial lining of the colon, is the predominant type (95% of cases) of colorectal cancer. When polyps become malignant, they extend into surrounding structures, invading and destroying normal tissues. These cancerous cells can migrate from the primary tumor and spread to other parts of the body, including the liver, peritoneum, and lungs. The symptoms of colorectal cancer depend on the tumor's location, the stage of the disease, and the function of the affected intestinal segment. Most people will report a change in bowel habits and blood in the stool. Other symptoms can include anorexia, weight loss, fatigue, and anemia. When the cancerous lesions are on the right side, symptoms usually include dull abdominal pain and melena (black, tarry stools). When the lesion occurs on the left side, people are more likely to have symptoms associated with intestinal obstruction (e.g., abdominal pain and cramping, narrowing stools, constipation, distention) and bright red blood in the stool. Finally, when the cancerous lesion is found in the rectum, symptoms can include tenesmus, rectal pain, blood in the stool, or alternating constipation and diarrhea. Complications associated with colorectal cancer can involve partial or complete bowel obstruction and hemorrhage due to ulceration in the surrounding blood vessels. These complications usually require surgical resection to create an ostomy (American Cancer Society, 2020b; Centers for Disease Control and Prevention [CDC], 2021; Hinkle & Cheever, 2018).
Ostomy Surgery of the Bowel
Ostomy creation is a surgical procedure that changes how intestinal contents leave the body by creating a purposeful anastomosis between the GI tract and the anterior abdominal wall. Whether temporary or permanent, ostomy surgery can be a lifesaving procedure for people with congenital disabilities, abdominal trauma, or medical conditions that damage the bowel. Fecal diversions or ostomies are indicated when the restoration of intestinal continuity is contraindicated or not immediately feasible given the person's clinical condition. A stoma is the opening on the abdomen created by ostomy surgery. The surgeon brings the small or large intestine through a hole in the abdominal muscle and connects it to the skin outside. A stoma may be between a three-quarter inch to two inches in width. Once the ostomy is created, the intestinal contents will leave the body through the stoma. Since a stoma has no muscle, the timing or flow of intestinal contents cannot be controlled. Therefore, a removable ostomy pouch or appliance is attached to the skin around the stoma to collect intestinal contents (see Figure 4). Fecal diversions are classified according to the segment of bowel used to create the ostomy (e.g., colon, ileum) and the manner of surgical construction (i.e., loop, end, reservoir; Francone, 2021; NIDDK, 2021b; United Ostomy Association of America [UOAA], n.d.-b).
Figure 4
Colostomy Bag and Stoma

Colon Resection
Surgical management of GI disorders does not always require the creation of an ostomy. Colon resection removes part of or the entire colon depending on the underlying etiology, including benign disease (i.e., Crohn's disease, colon polyps, colonic ischemia, fulminant colitis, colon trauma) or malignant colon lesions. Before surgery, the person should be thoroughly evaluated to identify comorbid conditions that increase the risk of postoperative morbidity and mortality. The type of resection performed can be open (i.e., laparotomy) or laparoscopic, depending on the extent and location of the diseased intestine. Benign disease can often be managed laparoscopically, whereas malignant disease is often performed as a laparotomy to resect the diseased intestine and the colonic vessels supplying blood to malignancy. The key to successful resection is ensuring adequate blood supply to the intestine (Lieske, 2021; Rodriguez-Bigas, 2020). Several systematic reviews and meta-analyses have evaluated open versus laparoscopic colectomy for people undergoing resection for diverticular disease, colitis, and colon cancer. Although laparoscopic surgeries may take longer than open surgeries, patients experience lower rates of perioperative morbidity and mortality, fewer complications, and no difference in long-term outcomes (Rodriguez-Bigas, 2020).
Regardless of the type or location of the resection, the surgical team should ensure the bowel proximal and distal to the resection is mobilized to ensure a tension-free anastomosis. In addition, the anastomosis should have a good blood supply (Lieske, 2021). There are many types of colon resections whose names are based on the anastomosis location (Fight Colorectal Cancer, n.d.; Rodriguez-Bigas, 2020):
- Segmental colectomy removes only the affected portion of the bowel.
- Ileocecetomy resects a portion of the distal ileum and the cecum.
- Right hemicolectomy removes a portion of the distal ileum, the cecum, ascending colon, and the transverse colon.
- Extended right hemicolectomy expands the right hemicolectomy to include the transverse colon.
- Transverse colectomy removes the transverse colon (see Figure 5).
- Left hemicolectomy removes the transverse colon to the left of the middle colic artery and the left colon and sigmoid colon to the level of the upper rectum.
- Sigmoidectomy removes the sigmoid colon.
- Total colectomy removes the entire colon.
Benign lesions are generally removed using a segmental resection or hemicolectomy (left or right). A segmental resection with a primary anastomosis in a well-vascularized colon can be performed for localized benign conditions (i.e., trauma, diverticular disease, IBD, polyp). A left hemicolectomy may be required for diverticular disease treated with a sigmoidectomy if the descending colon is unsuitable for an anastomosis. An extended resection (right or left) may be needed for more extensive benign diseases (i.e., IBD, diverticular disease, fulminant colitis). A right hemicolectomy can be performed for malignancy in the appendix, cecum, and ascending colon. A malignant lesion in the hepatic flexure or proximal to the mid-transverse colon can be resected with an extended right colectomy. A left hemicolectomy can be performed for a malignant lesion of the left colon (Rodriguez-Bigas, 2020).
Figure 5
Transverse Colectomy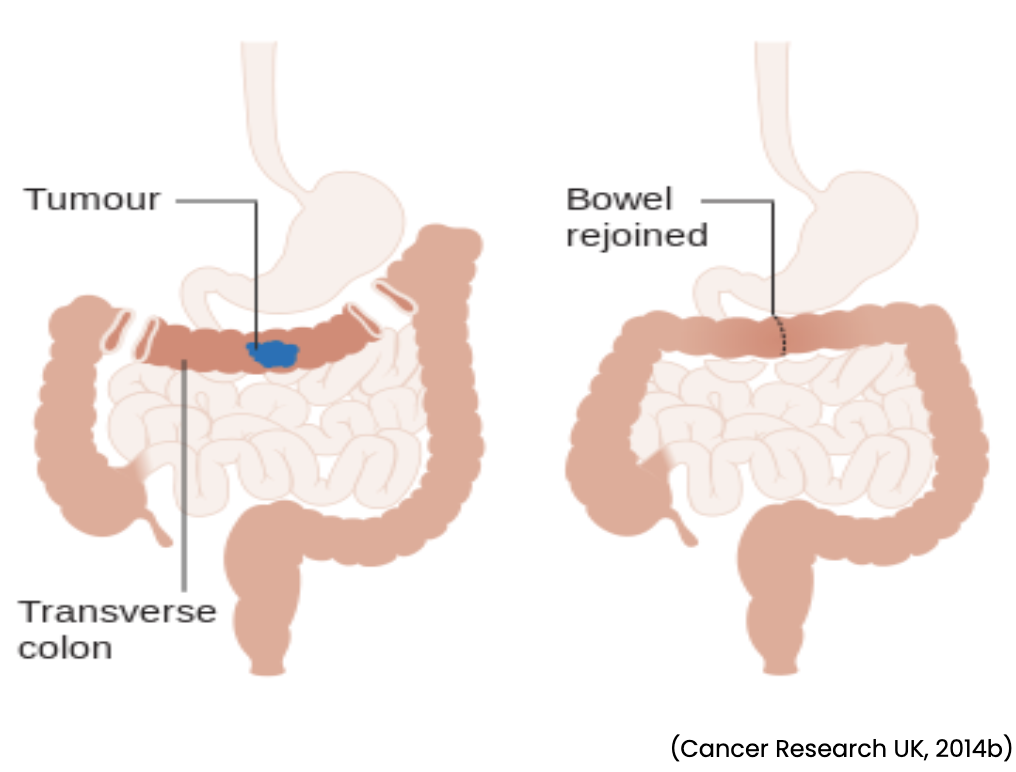
leostomy
An ileostomy is a surgical procedure during which the lumen of the small intestine (i.e., ileum) is brought through the abdominal wall to create a stoma. The purpose of an ileostomy is to evacuate stool from the body through the ileum instead of the anus; this is done when it is necessary to bypass the entire colon and rectum or to protect a distal colorectal, coloanal, or ileoanal anastomosis. An ileostomy can be placed temporarily or permanently and an end or a loop. The stool output of an ileostomy is loose due to the lack of water reabsorption that typically occurs in the large intestine. An ileostomy is typically created on the right side of the abdomen, and output ranges from 200 to 700 mL per day (see Figure 6). High-output ileostomies (i.e., greater than 1500 mL) increase the risk of electrolyte disturbances, dehydration, and malnutrition. Similarly, the location of the ileostomy should be as distal as possible to allow for maximum absorption of nutrients. The ileostomy is placed through the rectus abdominis muscle and sheath during the surgical procedure to reduce the risk of later parastomal hernia formation. Hernia formation can occur if the abdominal contents push through a weakness created by the surgical incision (Francone, 2021; NIDDK, 2021d; Rajaretnam & Lieske, 2021).
Figure 6
Ileostomy
Diverting Loop Ileostomy
In a loop ileostomy, a distal loop of the ileum is brought through the abdominal wall and then cut, creating 2 lumens draining into a stoma bag. This temporary fecal diversion is used for patients at high risk for an anastomosis leak (i.e., when stool passes through the joining of the ends of the bowel), on medications that may impair wound healing (e.g., steroids, immunosuppressive agents), or with a low colorectal anastomosis. A temporary loop ileostomy is preferred over a colostomy for protecting a distal colorectal anastomosis. With loop ileostomies, the proximal limb (afferent) passes the stool out of the stoma. In contrast, the distal limb (efferent) acts as a mucus fistula, draining secretions produced within the mucosal lining from the lumen to the cecum. The distal limb cannot drain colonic secretions if the ileocecal valve is intact. Therefore, for patients with a colonic obstruction, decompression of the colon cannot occur proximally or distally to the obstruction, likely resulting in a perforation. Once the distal anastomosis has healed, the proximal and distal limbs of the ileostomy can be joined back together, restoring the continuity of the GI tract. This ileostomy reversal is typically done 3 to 6 months after the initial creation (Francone, 2021; Rajaretnam & Lieske, 2021; UOAA, n.d.-a).
End Ileostomy
An end ileostomy is permanently created when there is no bowel to re-attach at a later stage. This is done when a total colectomy has been performed for colorectal cancer, Crohn's disease, colonic dysmotility, or UC. Additional indications include protection of a distal anastomosis and relief of a bowel obstruction. Brooke's technique is used for a permanent end ileostomy to create a stoma that extends 2 to 3 cm beyond the skin level. Brooke's technique involves the eversion of the bowel to expose the mucosa. Mucocutaneous suturing is then used to create the end stoma. This type of stoma promotes the secure application of the stoma appliance and adequate drainage into the pouch. In addition, the protruding stoma prevents skin irritation from the enzymatic drainage and the development of serositis and potential stricture (Francone, 2021; Rajaretnam & Lieske, 2021; UOAA, n.d.-a).
Continent Ileostomy
A continent ileostomy involves the creation of a continent ileal reservoir (i.e., Kock pouch) by diverting a portion of the distal ileum to the abdominal wall, creating a stoma. Then, approximately 30 cm of the distal ileum is utilized to form a reservoir with a nipple valve by pulling a portion of the terminal ileal loop back into the ileum. GI contents can accumulate in the pouch for several hours before removal by inserting a catheter into the nipple valve. This surgical procedure is rarely performed because specialized surgeons are needed. The continent ileostomy eliminates the need for an external fecal collection bag. Despite this advantage, many people require additional corrective surgeries for nipple valve malfunction. A diverting loop ileostomy is typically preferred because of the lower complication rate. A continent ileal reservoir is contraindicated for patients with Crohn's disease (Francone, 2021; Hinkle & Cheever, 2018; NIDDK, 2021d).
Restorative Proctocolectomy with Ileal Pouch Anal Anastomosis (IPAA)
A restorative proctocolectomy with IPAA is a surgical procedure for people with IBD whose rectum can be preserved because it eliminates the need for a permanent ileostomy. This procedure establishes an ileal reservoir that functions as a new rectum while anal sphincter control is retained (see Figure 7). During surgery, the ileum is connected to the anal pouch, which is made from a small intestine segment, while removing the colon and rectal mucosa (i.e., total abdominal colectomy and mucosal proctectomy). Then, a temporary diverting loop ileostomy is placed to allow the anastomoses to heal properly. A second surgery is performed to close the temporary ileostomy if the anal pouch heals properly (usually in 3 months). A restorative proctocolectomy with IPAA allows for voluntary defection and preservation of continence, making this option desirable for most people. Bowel movements can be more frequent than usual but gradually reduce over time. Complications of IPAA can include irritation of the perianal skin from the leakage of fecal contents, small bowel obstruction, pouchitis (i.e., inflammation of the ileoanal pouch), and stricture formation at the anastomosis site (Hinkle & Cheever, 2018; NIDDK, 2021d).
Figure 7
Ileoanal Reservoir
Colostomy
A colostomy is created by a surgical procedure when the end of the colon is brought through the abdominal wall to create a stoma. Like ileostomies, colostomies can be temporary or permanent and loop or end fashion. The choice of colostomy depends on the indication, the length of the mesocolon, the amount of diseased bowel, and the amount of remaining normal bowel. A colostomy is placed when there is a need to bypass or remove the distal colon, rectum, or anus. The need to bypass or remove part of the colon can occur from disease or obstruction. A colostomy is also indicated when it is not feasible or advisable to restore GI continuity using a colon resection. A colostomy can be placed in the ascending, transverse, descending, or sigmoid regions of the colon, with sigmoid and transverse being most common (see Figure 8). Ascending and descending colostomies are rarely performed. The stool becomes firmer by traveling along in the colon due to water and electrolyte absorption. For example, ascending and transverse colostomies lead to soft or loose, oatmeal-like stool. In contrast, descending colostomies produce a firmer, paste-like stool, and sigmoid colostomies create normal-resembling stool. A permanent colostomy is indicated when the distal rectum and anorectal sphincter mechanism are removed or when GI continuity cannot be restored. A permanent colostomy is preferable over an ileostomy due to the decreased risk of electrolyte abnormalities and dehydration (American Cancer Society, 2019c; Francone, 2021; Maria & Lieske, 2021).
Figure 8
Colostomy Types
Temporary Colostomies
A temporary colostomy can be performed emergently to decompress an intestinal obstruction or alleviate a perforated distal colon. This type of colostomy can also be done electively to facilitate the healing of perineal wounds, fistulas, or an acute inflammatory process in the distal bowel. If the distal rectum and anorectal sphincter mechanism are preserved, there is a potential for colostomy reversal once the distal anastomosis and colon have healed, typically 3 to 6 months after the creation. Different types of temporary, decompressing colostomies can be performed for patients with intestinal obstruction to evacuate fecal contents and promote bowel healing (American Cancer Society, 2019b; Francone, 2021; Mulita, 2021):
- A transverse loop colostomy is in the upper abdomen, either in the middle or toward the right side of the body. The transverse colon is brought through the abdominal wall, opened, and sutured to the dermis. Like the loop ileostomy, a loop colostomy has two openings—an opening for stool and another for mucus.
- A cecostomy is when the anterior wall of the cecum is opened and sutured to an opening in the abdominal wall.
- A sigmoid loop colostomy is similar to the transverse loop colostomy, utilizing the sigmoid colon. This procedure is helpful for obstructive rectal or anal cancer.
Although a diverting loop ileostomy is preferred to protect a colorectal anastomosis, a diverting colostomy can also be used. A colostomy can divert fecal content proximal to an area of inflammation (i.e., proctitis or perianal sepsis), unresectable distal malignancy, or a distal anastomosis. Fecal diversion can also be used for patients with failed fecal incontinence who are not candidates for other surgical interventions. Different approaches to temporary, diverting colostomies include the following (American Cancer Society, 2019b; Francone, 2021; Mulita, 2021):
- A loop colostomy is created with a proximal end (afferent limb) that allows fecal diversion and a distal end that allows venting of the distal length of the bowel (efferent limb). A loop colostomy minimizes the potential for perforation of the distal colon or rectum. Some indications for a loop colostomy include unresectable rectal cancer, radiation proctitis, incontinence, or perianal fistulas.
- An end-loop colostomy is created when there is a concern for ischemia of the bowel segment due to a foreshortened mesentery, allowing for additional length over an end stoma. Additional indications can include colon resection or perforated diverticulitis with peritonitis. This type of colostomy is created by exteriorizing a loop of the colon and dividing the colon with a linear stapler. This allows the distal end to recess into the subcutaneous space, using the proximal end to create the functional/active stoma.
- A double-barrel stoma is created by transecting the bowel and bringing each end to the abdominal wall. This creates two separate and adjacent stomas—a proximal functional colostomy and a distal mucus fistula. The end of the inactive part of the bowel can be sewn closed and left inside the abdomen (leaving a single stoma). The mucus from the resting part of the bowel exits through the anus.
- A Hartmann's procedure is created by bringing the proximal end of the transected bowel up to the abdominal wall as an end colostomy. Then, the distal end is oversewn or stapled and left in the abdominal cavity as a nonfunctional stump. The distal end can also be secured adjacent to the colostomy as a mucus fistula in the subcutaneous tissue but not matured to the skin.
Ostomy Reversal
Ostomy closure and reversal may be an option depending on the indication for ostomy placement, the type of ostomy performed, and the patient's clinical progress. For example, if the diseased bowel has improved or the anastomosis has healed, ostomy closure should be discussed. As discussed above, the distal rectum and anorectal sphincter mechanism must be functional for a reversal to occur. Ileostomy closure and reversal usually occur between 3 and 6 months when the anastomosis has healed (Francone, 2021). In a systematic review, Cheng and colleagues (2021) compared early versus late closure of an ileostomy following colorectal surgery. Early closure was defined as 8 to 17 days, while late closure was defined as 57 to 278 days across the five included studies. The researchers found that early closure required less operative time and reduced the incidence of a small bowel obstruction/postoperative ileus compared to late closure. However, early closure led to a higher incidence of surgical site infection and a longer length of stay. No difference was found for morbidity, reoperation, or anastomotic leak (Francone, 2021).
For diverting loop colostomies, closure and reversal can occur within 6 to 8 weeks of creation if the need for diversion no longer exists. For a temporary end colostomy, such as a Hartmann's procedure, closure is delayed until the resolution of the underlying condition (i.e., a return to baseline health status and softening of abdominal adhesions when inflammation subsides), usually in 3 to 6 months. For patients undergoing ostomy closure and reversal, HCPs should monitor for hernias. An estimated 1 in 3 patients who undergo ostomy reversal will develop an incisional hernia at the ostomy site. In addition, creating a temporary ileostomy or colostomy increases the chance of alterations in bowel function. Many patients will continue to experience rectal urgency, frequency, or incontinence after an ostomy closure and reversal (Francone, 2021; UOAA, 2018).
Wound, Ostomy, and Continence Nursing (WOCN)
WOCN is a nursing specialty that treats patients with acute and chronic wounds, ostomies, and complex incontinence conditions. Wound, ostomy, and continence (WOC) nurses hold a baccalaureate degree (BS) or higher and have completed additional education on caring for these complex patients. In addition to specialized training, nurses can become certified in WOC through the Wound, Ostomy, and Continence Nursing Certification Board. WOC nurses can provide acute, rehabilitative, and palliative support for GI, genitourinary (GU), and integumentary system disorders. In addition, they can administer direct, specialized care to people with stomas, wounds, fistulas, drains, pressure ulcers or injuries, and continence needs. WOC nurses focus on improving quality of care and QOL (Wound, Ostomy, and Continence Nurses Society, n.d.).
Regardless of the setting, WOC nurses can provide specialized ostomy care to maximize independence and adaptation to the life-altering changes in body image and function. Preoperative stoma site selection and marking are critical to successful stoma placement and management. Patients who receive preoperative education and site marking by a WOC nurse have fewer complications, increased independence, and increased QOL (Kozell et al., 2014). The WOCN Society and the American Society of Colon and Rectal Surgeons recommend that individuals undergoing fecal diversion have their stoma site marked by a colorectal surgeon or a certified WOC nurse (Hendren et al., 2015). Immediately after the ostomy surgery, the WOC nurse should be consulted to select and fit the ostomy pouching system. Choosing the appropriate device requires WOC nurses to consider the patient's unique medical and physical needs, preferences, and QOL. When a WOC nurse is consulted in the immediate postoperative period, the risk of ostomy-related complications is decreased. Long-term support and specialized ostomy care are needed for patients with permanent ostomies. WOC nurses can facilitate participation in support groups and advocate for coverage of ostomy supplies (Wound, Ostomy, and Continence Nurses Society Task Force, 2018).
Preoperative Preparation for Ostomy Surgery
Preoperative preparation is critical for minimizing postoperative complications and preparing each patient for life-altering ostomy surgery. Patients who require an ostomy can experience physical, psychological, and emotional stress related to social acceptance, sexuality, and economic burden. Therefore, when possible, HCPs should begin preoperative patient education, counseling, and emotional support as soon as the need for an ostomy is determined. In addition, preoperative preparation should include stoma site selection and early planning for discharge, rehabilitation care, and outpatient follow-up. Although various HCPs can perform these preoperative preparation activities, utilizing the specialty skills of a WOC nurse is preferred. Preoperative counseling by a WOC nurse improves postoperative QOL, decreases the length of stay, and reduces the risk of complications (Francone, 2021).
Regardless of who completes the preoperative preparation, the following criteria should be addressed.
- An individualized preoperative and postoperative patient education plan should be developed. Every patient will approach ostomy surgery differently. Ostomy surgery can be lifesaving; however, initial feelings of fear and negativity can affect some patients. For other patients, ostomy surgery can mean the end of years of unresolved incontinence or IBD. The new ostomy will require long-term management and care. HCPs will need to determine each patient's level of independence with ostomy care and decide whether other support services are needed (American Society of Colon & Rectal Surgeons [ASCRS], n.d.; Francone, 2021; Landmann & Cashman, 2021; NIDDK, 2021e).
- The patient should meet with a WOC nurse prior to surgery when possible. The WOC nurse can provide comprehensive education and facilitate pouch-changing practice before surgery. In addition, the WOC nurse can assist the patient in locating a UOAA-affiliated support group that offers ongoing education, resources, and emotional support to patients before and after ostomy surgery. Several retrospective studies have confirmed that involvement In an ostomy support group improves long-term patient outcomes and reduces the risk of complications (ASCRS, n.d.; Francone, 2021; Landmann & Cashman, 2021; NIDDK, 2021e).
- Preoperative stoma site selection should be completed. As mentioned earlier, when a colorectal surgeon or WOC nurse performs the preoperative stoma site selection, patients experience fewer ostomy-related complications, improved independence, and reduced healthcare costs. Poor site selection is more likely to occur in emergent versus elective procedures. When considering the appropriate site, HCPs should ensure the stoma site can be easily reached, is less likely to get in the way of activities or clothing, and reduces the risk of complications. The HCP should examine the patient in many different positions (i.e., standing, sitting, and lying down) to help determine the best location. Most stoma sites will be in the lower part of the abdomen, just below the beltline. However, for patients with a higher body-mass-Index (BMI), the stoma may need to be placed in the upper abdomen. In addition, HCPs should assess each patient’s abdominal wall contour, prior abdominal incisions, bony prominences, occupation, disability, and physical limitations (ASCRS, n.d.; Francone, 2021; Landmann & Cashman, 2021; NIDDK, 2021e).
- Enhanced recovery after surgery (ERAS) protocols highlight the importance of optimizing the patient's functional status before surgery. For example, the consumption of alcohol, smoking, and recreational drugs should be discouraged. Optimizing the patient's functional status before surgery can decrease the risk of post-surgery morbidity (ASCRS, n.d.; Francone, 2021; Landmann & Cashman, 2021; NIDDK, 2021e).
During the immediate preoperative period, HCPs should anticipate the following considerations for ostomy surgery preparation. First, preoperative bowel preparation is a common practice before a bowel procedure or surgery. A cleansed colon facilitates bowel manipulation, enables the passage and firing of surgical staplers, and allows for intraoperative colonoscopy. The role of bowel preparation in reducing surgical morbidity and surgical site infections (SSI) has been heavily studied and debated. Colon and rectal surgeries have the highest rates of SSIs of all elective surgeries due to the high bacterial count in the colon. Mechanical bowel preparation (MBP) has been used to decrease the intraluminal bacterial concentration and reduce SSIs. Bowel preparation can include administering an MBP in conjunction with oral antibiotics. The most commonly used oral cathartics for MBP include sodium phosphate (OsmoPrep) and polyethylene glycol (MiraLax). The oral antibiotic regimen used in MBP varies across studies, with the most common regimen being neomycin (Mycifradin) and metronidazole (Flagyl). Several recent studies have shown reduced SSIs with bundles that include MBP and oral antibiotics. According to the ASCRS, MBP combined with preoperative oral antibiotics is typically recommended for elective colorectal resections to reduce the rates of SSIs, anastomotic leak, readmission, and length of stay (Migaly et al., 2018). Next, a nasogastric (NG) tube may be placed in the preoperative or intraoperative period for bowel decompression. In addition, a foley catheter must be placed to keep the bladder empty during surgery to prevent an intraoperative injury and monitor urine output. Finally, adequate fluid and electrolyte resuscitation can prevent dehydration during surgery (Maria & Lieske, 2021; Mulita, 2021).
Postoperative Ostomy Management and Care
For any patient undergoing colon resection, ileostomy or colostomy placement, or ostomy reversal, HCPs will need to provide postoperative care as per general abdominal surgery. General abdominal surgery postoperative considerations include routine assessments of vital signs, head-to-toe assessments (especially for bowel sounds and flatus production), and pain management. As with other patients undergoing abdominal surgeries, HCPs should encourage early mobilization to prevent postoperative complications, including deep vein thrombosis (DVT) and pneumonia. In addition, patients in the immediate postoperative period are at risk for dehydration or hypovolemia from blood and fluid losses during surgery. In the immediate postoperative period, additional daily fluid losses from fecal drainage, urine, perspiration, and other sources can be as much as 1 L to 2 L. Therefore, the HCP should monitor and document the patient’s intake and output and laboratory values (i.e., hemoglobin and hematocrit, electrolytes including sodium and potassium, and kidney function). Fluid volume replacement utilizing intravenous (IV) solutions is typically administered for 4 to 5 days after surgery. If the patient has an NG tube placed before or during surgery, it will remain in place to prevent the buildup of gastric contents while the intestines are not working. The HCP will need to ensure the NG tube is hooked up to suction and monitor, assess, and document the drainage. After the NG tube is removed, the patient's diet will gradually be progressed, starting with small sips of clear liquids. The HCP should assess the stoma (described below) and the ostomy for fecal drainage, which should begin 24 to 48 hours after ileostomy surgery and within 3 to 6 days after colostomy surgery (Hinkle & Cheever, 2018).
Stoma Creation and Management
For patients with an end stoma ileostomy, the stoma is created by resecting the small intestine and bringing the end through the abdominal wall. Then, the stoma is inverted and sewn into place. In contrast, a loop stoma is created by bringing a loop of the small intestine through the abdominal wall. Next, the bowel is opened, creating a proximal and a distal tract. Finally, the open edges are everted and sutured to the skin. Loop stomas are supported in position by a bridge/rod of plastic or rubber that prevents the stoma from retracting. The bridge/rod will usually remain in place for 2 to 7 days (Berti-Hearn & Elliott, 2019b). The stoma construction can be an end, loop, or double barrel for patients with a colostomy. Like an ileostomy, the loop stoma for a colostomy will have a bridge for stomal support. Double barrel stomas are rarely seen and have two stomas resembling end stomas (Berti-Hearn & Elliott, 2019a). The HCP should assess the stoma (i.e., color, protrusion, edema, and size) and any incisions for drainage or signs of infection. The stoma should be pink to bright red, moist, and shiny and extend about 1 to 3 cm beyond the skin level (see Figure 9). As the stoma matures, it will change in size and occasionally shape (an ileostomy stoma is usually smaller than a colostomy stoma). Once the stoma matures, it should have a soft contour and appear flush with the skin or slightly protruding above the skin. Each time the pouch is changed (usually once or twice a week), the HCP should measure the stoma and adjust the opening in the skin barrier as needed. The peristomal skin should look like the rest of the skin on the abdomen and be clean, dry, and intact. If the pouching system does not fit correctly, ostomy drainage (i.e., effluent) can damage the peristomal skin. See details on pouching systems and routine ostomy care below for additional care and management (Berti-Hearn & Elliott, 2019b; Stelton, 2019).
Figure 9
Ileostomy Stoma
Ostomy-Related Complications
HCPs must be aware of the various complications that can occur when a patient has an intestinal ostomy. Nearly half of all stomas eventually develop complications due to stoma and peristomal skin issues. Complications can be early (i.e., occurring within days to 3 months after placement) or late (i.e., occurring after 3 months) and can vary based on the type of ostomy. For example, end colostomies (ileostomy or colostomy) have the fewest complications. In contrast, loop ileostomies have the most complications, such as dehydration from high-output, skin irritation from high alkaline enzymatic effluent, and small bowel obstruction. Stomal prolapse (see figure 10) is most prevalent in transverse loop colostomies. Peristomal hernia and retraction (see figure 10) are the primary complications for both end and loop colostomies and ileostomies. Very early complications (i.e., occurring within days of ostomy surgery) can include a large bowel obstruction due to a twist in the bowel leading to the stoma. Very early complications usually require a second surgery (Landmann & Cashman, 2021).
Figure 10
Stoma Complications
Early Postoperative Stoma Complications
Early complications (i.e., occurring within 3 months after stoma construction) can include stomal edema, stomal ischemia/necrosis, stomal bleeding, stomal retraction, and mucocutaneous separation. These complications are usually related to suboptimal stoma site selection or high-risk patient factors (i.e., old age, poor nutritional status, comorbidities, obesity, tobacco use, or underlying cancer; Landmann & Cashman, 2021). Stomal edema is a common occurrence immediately after surgery but should subside over 6 to 8 weeks. When edema is present, the stoma will have a translucent, fluid-filled appearance and be light pink. As the edema subsides, the characteristic dark pink to red color will return. An edematous stoma is fragile, and bleeding can easily occur during skin cleansing and pouch application or removal. Therefore, when applying the skin barrier for the pouch, the HCP should cut a circle that is slightly larger than the usual size to accommodate additional edema (Stelton, 2019).
Stomal ischemia and necrosis can occur for various reasons, including emergency surgery, IBD, abdominal distention, and a small opening in the fascia or muscle. In addition, patients with a higher BMI are at increased risk for stomal ischemia due to thicker abdominal walls and greater tension exerted on the bowel. The incidence of stomal necrosis in the immediate postoperative period is as high as 14%. When blood flow is impaired, the stoma will appear less shiny and burgundy or purple. Prolonged impairment in circulation to the stoma can lead to ischemia, necrosis (i.e., tissue death), and skin sloughing. If tissue sloughing does occur, the skin should not be peeled off the stoma. Stoma viability should be frequently assessed in the first few days after surgery (i.e., color, temperature, moistness, tissue turgor). If impaired circulation is suspected, the surgeon should be notified immediately (Stelton, 2019).
Minor stoma bleeding is expected immediately after surgery due to the vascular supply to the bowel. A small amount of sanguineous drainage (i.e., a few drops) is normal and usually subsides within a few days. However, a steady drip or flow of blood from the stoma is not normal and should be brought to the surgeon’s attention immediately. Major bleeding could indicate a stomal laceration, a poorly fitting appliance, or the presence of peristomal varices in patients with portal hypertension (usually those with UC). If minor bleeding is noted, applying a cool cloth with light pressure should stop the bleeding. Other treatment options could include local cauterization (i.e., handheld cautery or silver nitrate) or suturing the bleeding vessel if it is easily identified (Landmann & Cashman, 2021; Stelton, 2019).
Stomal retraction (see figure 10) occurs when the stoma retracts to 0.5 cm or more below the skin surface. This retraction happens within 6 weeks of construction due to tension on the stoma. The incidence of stomal retraction ranges from 1% to 40% and can lead to leakage, pouch-adherence problems, and peristomal skin irritation. This risk for stomal retraction increases in patients with obesity or when the initial stoma height is less than 10 mm (Landmann & Cashman, 2021).
During the surgical placement of an ostomy, the stoma edges are secured to the surrounding skin with sutures. Mucocutaneous separation (i.e., stoma dehiscence) occurs when the stoma detaches from the peristomal skin and affects 12% to 24% of patients in the early postoperative period. The separation can involve 1 or 2 sutures or appear around the entire stoma. For minor separations, the space can be filled with pectin-based stoma powder or a small piece of absorbent dressing (i.e., calcium alginate, silver alginate). Then, the pouch is applied over the separated area. Severe separations involve the entire perimeter of the stoma and can lead to stoma retraction below the fascia level and effluent leakage into the abdominal cavity. Patients who have diabetes, poor nutritional status, or received high-dose steroids or chemotherapy before surgery are at higher risk for mucocutaneous separation. HCPs should notify the surgeon immediately if mucocutaneous separation occurs (Landmann & Cashman, 2021; Stelton, 2019).
Late Stoma Complications
Late stoma complications, occurring 3 months after surgery, are usually related to permanent ostomies and include stomal prolapse, stomal stenosis, and parastomal hernia. Factors that increase the likelihood of late complications include the length of time the stoma has been present, increased intra-abdominal pressure (e.g., due to obesity, chronic obstructive pulmonary disease [COPD]), emergency surgery, and a stoma height of less than 10 mm. Stoma prolapse (see figure 10) occurs when additional bowel tissue protrudes through the stoma for loop stomas and patients with abdominal muscle weakness (e.g., those who are elderly or malnourished). The incidence of stomal prolapse is approximately 7% to 26%. If the stoma functions well and the bowel tissue is pink to red and moist, the prolapse can be considered non-urgent. Stomal prolapse can make applying the pouching system more challenging. Having the patient lie flat or applying a cool cloth to the prolapsed bowel can reduce the size of the prolapse, allowing for easier application. Convex pouches and ostomy appliance belts should be avoided for patients with stomal prolapse. Manual reduction of the prolapse and application of a binder are conservative approaches to managing non-urgent stomal prolapses. However, prolonged prolapses can lead to intestinal edema and strangulation. Stomal prolapse requires surgical intervention if bowel ischemia or severe mucosal irritation manifests (Landmann & Cashman, 2021; Stelton, 2019).
Stomal stenosis (see figure 10) occurs when the opening shrinks to a small diameter, usually interfering with normal function. The incidence of stomal stenosis is 2% to 15%, and most cases involve end-colostomies. Although stomal stenosis can appear in the early postoperative period, it is more likely to occur months later. Early stenosis with an ileostomy can occur due to edema at the fascial and superficial levels. This type of stenosis can be managed by inserting a 36 French soft-tipped foley catheter into the stoma, just proximal to the fascial level. The balloon should not be inflated, and insertion should not be attempted if significant resistance is met. Late stenosis can occur at the skin or fascial level due to scarring or tightness of the mucocutaneous junction. Other causes of stomal stenosis include mucocutaneous separation, stoma necrosis, retraction, peristomal sepsis, or an ill-fitting pouching system. Minor stomal stenosis can be conservatively managed with a low-fiber diet. More complex stenosis, or stenosis of a colostomy, can be problematic if stool cannot be evacuated. These complex cases may require surgical intervention (Landmann & Cashman, 2021; Stelton, 2019).
A peristomal hernia (see figure 10) occurs when additional intestinal tissue protrudes through the muscle layer, creating a bump or bulge. These hernias are relatively common, particularly among colostomy patients. Risk factors for hernia development include placement of the stoma outside of the rectus muscle, obesity or poor abdominal muscle tone, chronic coughing, and a sizeable fascial opening. If the stoma is still functioning normally and is pink to red, then the hernia is not emergent. However, the hernia can impact the fit of the pouching system, requiring more frequent changes. A one-piece pouch can provide a more flexible fit over the hernia, and a support belt may also help. If the stoma becomes bluish or the patient experiences abdominal pain, a lack of stool output, or vomiting, they should be evaluated immediately (Landmann & Cashman, 2021; Stelton, 2019).
Peristomal Skin Complications
Peristomal skin breakdown (see figure 10) is the most common ostomy-related complication, occurring in the early and late postoperative periods. As many as 45% of ostomies can have short-term or long-term peristomal skin complications. These are more likely to occur with ileostomies and can vary in severity from minor skin trauma to dermatitis and pyoderma gangrenosum (PG). When possible, the patient should be evaluated by a WOC nurse when peristomal skin problems manifest. Mechanical trauma (i.e., skin-stripping) occurs from repeatedly removing adhesive products or overly aggressive cleansing techniques. These mechanical injuries usually involve the removal of the epidermis and present as patchy areas of irritated, denuded skin. Patients with peristomal hair should be taught to clip the hair to prevent follicle trauma. Before placing a new pouch, the patient should apply pectin-based ostomy powder to the superficial wounds. A non-alcohol-based skin protectant wipe or spray can be used before pouch placement with an adhesive remover before pouch removal to prevent skin-stripping or mechanical trauma (Landmann & Cashman, 2021; Stelton, 2019; Wound, Ostomy, and Continence Nurses Society Peristomal Skin Complications Task Force, 2015).
Irritant dermatitis is the most common peristomal skin complication caused by stoma effluent coming in contact with the skin. This dermatitis is more common with ileostomies than colostomies due to the digestive enzymes and electrolyte content of the effluent. The severity of dermatitis can range from minor erythema to blisters, usually related to how long the effluent was in contact with the skin. If a pouching system is leaking, the HCP should not tape the edge because this traps the effluent onto the skin. Instead, the pouching system should be changed. When dermatitis is present, a protective barrier wipe should be used to make the skin sticky, and a pectin-based ostomy powder should be applied (Landmann & Cashman, 2021; Stelton, 2019).
Allergic dermatitis occurs when the peristomal skin becomes inflamed due to an allergy to any skin barrier pouching system component, usually the adhesive substance in the tape. Dermatitis generally presents with redness, blisters, or weeping tissue. A topical steroid spray or a small amount of steroid cream can be applied for allergic dermatitis (Landmann & Cashman, 2021; Stelton, 2019).
Peristomal pyoderma gangrenosum (PPG) is a subtype of PG, a neutrophilic dermatosis (full-thickness lesions or an ulcer with a bluish-purple discoloration at the wound edges) with an unclear etiology. Although PPG affects the peristomal skin, it is not caused by stoma effluent or mechanical injury. Instead, PPG is a symptomatic exacerbation of a systemic chronic inflammatory disease (e.g., Crohn's disease, rheumatoid arthritis, or lupus). PPG occurs at the stoma site, typically in patients with IBD. The overall incidence of PPG is 2% to 4%, and it can develop within weeks to years after stoma construction. Most PPG patients are female (67%), with a mean age of 48, and have a diagnosis of IBD (50% with Crohn's disease and 31% with UC). There is no definitive diagnosis for PPG; therefore, the diagnosis is based on exclusion. Since PPG is rare, it is often misdiagnosed as a stitch abscess, contact dermatitis, a urinary or fecal fistula, an extension of Crohn's disease, or wound infection. If PPG is suspected, the patient should be referred to dermatology for a biopsy to rule out other causes, such as Crohn's disease or cancer (Landmann & Cashman, 2021; Stelton, 2019).
In addition to mechanical trauma and dermatitis, other peristomal skin problems can include parastomal ulceration, granulomas, folliculitis, and peristomal hyperplasia. Parastomal ulceration occurs when there is discontinuity of the peristomal skin with adjacent inflammation. This is usually due to an infected postoperative hematoma or intestinal fistula. Granulomas are red, moist, elevated lesions that develop at the mucocutaneous border due to retained sutures or other extraneous material. These granulomas may be tender and can easily bleed or become infected. Treatment includes removing the extraneous material, and silver nitrate should be applied to eliminate any elevated tissue. Folliculitis occurs when the hair follicles under the adhesive of the pouching system become inflamed pustules. Folliculitis is more common in men and can be prevented by periodic hair removal, either by plucking or shaving with an electric razor. Finally, peristomal hyperplasia (i.e., pseudoverrucous lesions) is bumpy tissue that arises from a buildup of epithelial tissue around the stoma. Hyperplasia occurs due to chronic inflammation of the peristomal skin caused by exposure to leaking stoma effluent or when the pouch barrier is cut too large. If hyperplasia occurs, applying a pouch can be challenging; silver nitrate can be applied to cauterize bleeding and reduce the size of the lesions (Landmann & Cashman, 2021; Stelton, 2019).
Other Ostomy-Related Complications
For patients with an ileostomy, digestive enzymes in the effluent can irritate the mucosa and skin, which increases the risk of stomal and peristomal skin complications discussed earlier. In addition, ileostomies produce effluent ranging from 500 mL to 1300 mL per day, increasing the likelihood of dehydration. Up to 30% of patients will develop dehydration after loop ileostomy creation; therefore, patients should be encouraged to consume at least 2 to 3 L of fluid per day. Fluid intake should be increased even more during high-volume output or sweating periods. HCPs should educate patients regarding appropriate fluids to consume, such as water, broth, vegetable juices, and pediatric electrolyte solutions (i.e., Pedialyte, Emergen-C). Sports drinks should be avoided because they may not be absorbed, and some can exacerbate stoma output. Patients with ileostomies should also be taught about signs and symptoms of electrolyte imbalances since the effluent contains significant amounts of sodium and potassium. Symptoms of electrolyte imbalances can include a dry mouth, reduced urine output, dark-colored urine, dizziness upon standing, fatigue, and abdominal cramping. Protocols and postoperative clinical pathways can minimize dehydration and prevent readmissions for patients with ileostomies (Landmann & Cashman, 2021).
Another complication associated with ileostomies is high-ostomy output (see figure 10), defined as greater than 1.5 L per day. Periods of high-ostomy output can increase the risk of dehydration and other stomal and peristomal skin complications. First-line therapy for high-ostomy output includes soluble fiber supplementation (i.e., polycarbophil [FiberCon] and wheat dextrin [Benefiber]) to slow the transit time, allowing for water absorption. Patients may slowly increase supplementation up to 4 times a day until a gel-like consistency in the output is formed. Insoluble fibers (i.e., Citrucel [methylcellulose]) are bulk-forming and should be avoided because they can speed transit time. For patients whose high output does not resolve with increased soluble fiber supplementation, other treatment options include antimotility agents (i.e., loperamide [Immodium], diphenoxylate, and atropine [Lomotil], octreotide [Sandostatin], and cholestyramine [Questran]). Loperamide is the preferred first-line antimotility agent because it is available over-the-counter (OTC) and has few side effects. Patients should start with a single tablet BID or TID based on stoma output, and dosing can be increased slowly. Antimotility agents should not be combined due to the risk of paralytic ileus or obstruction. Patients with high-output ileostomies will need frequent pouching system changes and a protective skin barrier (Landmann & Cashman, 2021).
Ileostomy patients are also at risk of food blockage and drug malabsorption. Food blockage can occur because the ileal lumen is less than 1 inch in diameter, with further narrowing as the bowel passes through the fascia/muscle layer. In addition, if patients consume large amounts of insoluble fiber, the undigested fiber can form an obstructing mass (i.e., bezoar). Therefore, patients should be taught to limit foods such as popcorn, mushrooms, black olives, coconut, stringy vegetables, corn, nuts, celery, foods with skins, dried fruits, and meats with casings. They should consume these foods one at a time in small quantities, chewing thoroughly and monitoring their response. Since most drug absorption occurs in the small bowel, patients with ileostomies are at risk for suboptimal absorption. Patients should take medications in quick-dissolve forms such as liquids, uncoated tablets, or gelatin capsules. Time-released and enteric-coated medications should be avoided (Landmann & Cashman, 2021).
For patients with descending/sigmoid colostomies, the stool is formed and does not contain digestive enzymes; therefore, there is a decreased risk of stomal and peristomal complications. Although patients with colostomies do not have dietary restrictions, they should consume adequate fiber (soluble and insoluble; 20 to 35 grams per day) and fluid (1.5 to 2 liters per day) to prevent constipation. If constipation does occur, patients can be managed with laxatives or colon irrigation. Colon irrigation involves instilling 500 mL to 1500 mL of tap water into the descending or sigmoid colon ostomy on a routine schedule (i.e., daily or every other day). The instilled tap water distends the bowel to stimulate peristaltic activity, causing the distal colon to empty. Colon irrigation should only be done for patients with distal colostomies. Patients who manage their colostomies with routine irrigation can be considered continent; therefore, they may only need a small pouch or cap for mucus collection and deodorization of flatus. Constipation and the risk for postoperative ileus in the first few days after surgery can be minimized with early mobilization and following an ERAS protocol (Landmann & Cashman, 2021).
Pouching Systems
After ostomy creation, the HCP needs to provide peristomal skincare and choose a pouching system (i.e., products used to collect stool) that will work best for the patient. The skin barrier includes a sticky backing that adheres to the skin, while the pouch holds the stool (see Figure 11). A WOC nurse should be consulted for advice on the appropriate pouching system when possible. Many factors should be considered, including the type of ostomy, stoma type and location, patient lifestyle, abdominal contours, patient preference, visual acuity, and manual dexterity. In addition, the pouching system should fit appropriately and maintain a tight seal to prevent leakage. Poorly fitting pouching systems and effluent leakage contribute significantly to stomal and peristomal complications. Finally, a good pouching system should also be odor resistant, be nearly invisible under clothing, be easy to put on and take off, and allow the patient to shower or bathe with the pouch in place (American Cancer Society, 2019a; Wound, Ostomy, and Continence Society Guideline Development Task Force, 2018).
Figure 11
Ostomy Skin Barriers
An ostomy pouching system has many functions, including collecting effluent, containing odor, and protecting the peristomal skin. Pouching systems come in many different styles and sizes, but all have a pouch to collect stool drainage and an adhesive barrier (i.e., flange, skin barrier, or wafer, as shown in Figure 12). There are two main types of pouching systems: one-piece and two-piece. With a one-piece system, the pouch and the adhesive barrier are attached; when the pouch is removed, so is the barrier. In contrast, a two-piece system (see Figure 12) has a pouch separate from the adhesive barrier; the barrier remains in place when the pouch is taken off. A one-piece system offers simplicity, while the two-piece system allows the pouch to be changed without removing the skin barrier. Some pouching systems can be opened at the bottom for emptying, while others can be taken off when full (see Figure 13). The choice of an open versus a closed pouch depends on insurance coverage and the number of stools per day (for more than two, an open pouch is preferred). Patients with an ileostomy should use an open pouch due to the frequency of emptying. Some systems allow the adhesive barrier to remain in place while the pouch is removed, cleaned, and reused (see Figure 14). Pouches are designed to be odor-resistant, can be clear or opaque, and vary in length. Patients with a colostomy that produces stool at regular, expected intervals can use a stoma cap instead of a pouch. To create a stoma cap, place a small piece of gauze dabbed with a small amount of water-soluble lubricant over the stoma. Cover this with a piece of plastic wrap and medical tape. Plastic, ready-made stoma caps can also be used (American Cancer Society, 2019b; Berti-Hearn & Elliott, 2019a, 2019b).
Figure 12
Ostomy Wafer Covering an Ileostomy

The opening of the adhesive skin barrier needs to be sized appropriately to fit the stoma (see Figure 15). The opening should be no more than 1/8 inch larger than the stoma to protect the peristomal skin from the stoma output. Some pouching systems require the stoma hole to be cut, while others come pre-cut. Due to swelling, the stoma size can change for about 6 to 8 weeks post-surgery. Therefore, the stoma should be measured once a week during this time to ensure accurate sizing for the adhesive barrier (American Cancer Society, 2019b). For pouching systems with a flat and rigid adhesive barrier, a flat abdominal area (i.e., without scars, skin creases or folds, and bony prominences) helps prevent complications. A convex pouching system should be considered if the stoma is located on a concave abdominal plane. Additional adjunctive products can promote pouch fixation to the skin (i.e., adhesive agents, Skin Prep) and prevent peristomal skin injury. Skin barrier paste, powder, or rings should be used consistently for patients with a loop ileostomy. The patient will need to choose a reliable, durable medical equipment supplier to obtain their supplies at home (Berti-Hearn & Elliott, 2019a, 2019b; Landmann & Cashman, 2021).
Figure 15
Ostomy Pouch Placement
 Routine Ostomy Care
Routine Ostomy Care
During the postoperative period, the HCP or WOC nurse should teach the patient or caregiver how to provide routine ostomy care in preparation for discharge home. The frequency of performing ostomy care and changing the appliance will vary depending on the specific appliance and needs of the patient but is usually once or twice per week. The length of time that the pouch will stay sealed depends on several factors such as weather, skin condition, weight changes, scars, activity, diet, and the nature of the output. Before starting the procedure, all necessary supplies should be gathered, and handwashing should be performed. The pouch should be emptied of all content before removing the adhesive skin barrier. An adhesive remover may be required. Next, the stoma and peristomal skin should be gently cleaned with water, using a dabbing—not scrubbing—technique. The HCP should assess the stoma and peristomal skin for any signs of the complications discussed above. Next, the peristomal skin should be dried to ensure a good seal for the new skin barrier appliance. Adhesive pastes or powders may be applied to the peristomal skin as needed. The paper cover on the back of the adhesive skin barrier is removed, placed around the stoma, and held in place for 1 to 2 minutes to ensure an adequate seal. For two-piece systems, the pouch will then be clipped onto the appliance (Maria & Lieske, 2021).
Patients should be instructed to empty the pouch when it is approximately one-third to one-half full to prevent disruption of the pouch seal due to excessive weight. The patient should be taught to sit as far back on the toilet as possible. An alternative position could be in a chair facing the toilet. Hold the bottom of the pouch upward and open the clip on the end of the pouch. Unroll the tail and evert it to keep it clean; keeping this part clean will prevent stool from getting on the tail and causing odor. Tail eversion is not necessary for pouching systems with an integrated closure mechanism. Gently empty the contents and then clean the outside and inside of the tail. Roll the tail and clip the end of the pouch. Patients can use a pouch deodorant or room spray to minimize odor during pouch emptying. If the patient is overly concerned about stool odor, bismuth subgallate and chlorophyllin copper complex can reduce stool odor. Bismuth subgallate should be used for patients with ileostomies or proximal colostomies because it can thicken stool. Chlorophyllin should be used for descending or sigmoid colostomies because it can have a diarrheal effect (American Cancer Society, 2019a; Landmann & Cashman, 2021; UOAA, 2020).
Ostomy-Related Patient Education
Patients with a newly created colostomy or ileostomy must adapt to alterations in their body and new patterns of fecal elimination. Successful adaptation requires each patient to deal with the emotional and psychological aspects of ostomy creation and master new ostomy care skills. Patients may experience stages of grief (i.e., denial, anger, bargaining, depression, or acceptance) post-surgery, and these emotions can affect retention of postoperative discharge education. HCPs need to support patients through these stages using a calm, nonjudgmental attitude to instill self-confidence. In addition, support groups and family engagement can help patients adjust to this new way of life. Patients and caregivers will have many questions about the new colostomy or ileostomy and its impact on daily life. Providing verbal, written, and hands-on information is essential for successful discharge to home with a new ostomy. This education should be started while the patient is recovering in the hospital post-surgery. The HCP should assess the patient's manual dexterity to determine if they can perform ostomy care independently. Homecare clinicians will continue this education once the patient has been discharged home until they can manage their ostomy independently. Patients with a newly placed ostomy are often discharged home 5 to 7 days after surgery (Hinkle & Cheever, 2018; Landmann & Cashman, 2021).
A comprehensive discharge plan for a patient with a new ostomy includes teaching skills for changing the pouching system, cleaning the peristomal skin, and emptying the pouch, as discussed above. Additional patient education should include signs and symptoms of complications, dietary and fluid considerations, medication management, and physical activity (see Table 2). Patients will also need assistance ordering supplies and setting up home care services (Wound, Ostomy, and Continence Nurses Society, 2014).
HCPs should educate patients about the following signs and symptoms of potential complications to report to the surgeon:
- Inability to establish a pouch seal for a predictable amount of time
- Peristomal skin changes (i.e., rash, lesion, or denuded skin) that does not heal after 1 to 2 pouch changes
- Separation of the stoma and peristomal skin
- Change in color of the stoma (i.e., dark purple or black indicates necrosis; white or yellow linear discoloration indicates trauma)
- Change in the length or appearance of the stoma (i.e., prolapse or protrusion, retraction, or narrowing of the lumen)
- Prolonged abdominal pain, nausea, vomiting, or abdominal distention
- Change in stomal output or function (e.g., blood in stool, no colostomy output for 2 days, no ileostomy output for 8 hours)
- Excessive thirst, weakness, or concentrated urine (Wound, Ostomy, and Continence Nurses Society, 2014)
After successful ostomy surgery, most patients will not have specific dietary restrictions. However, patients will need to maintain a well-balanced diet that includes adequate protein, vitamins, and minerals. In the weeks after surgery, many patients do not have a large appetite; therefore, six small meals per day should be encouraged to ensure adequate calories and absorption. Patients with ileostomies experience the most dietary challenges. These patients should initially avoid raw fruits and vegetables and then slowly add fiber, cooked vegetables, and fruit to their diet. Foods should be introduced individually to monitor for which foods cause gas, odor, or loose stools. Patients with an ileostomy should avoid high-fiber, stringy, and non-digestible foods (e.g., corn, celery, asparagus, nuts, coconut, popcorn, mushrooms) due to the risk of food blockage. Additional postoperative dietary education for ostomy patients can include the following (Berti-Hearn & Elliott, 2019a, 2019b; Mayo Clinic, 2020):
- Consume 2 to 3 L of fluid daily to prevent dehydration.
- Fiber consumption is essential for colostomy patients. Patients should be encouraged to eat a well-balanced diet with fruits and vegetables. Fiber can cause gas, so it should be introduced gradually into the diet. Fiber supplements may be needed.
- Patients should be encouraged to eat slowly and chew thoroughly to avoid gas. In addition, avoid straws, chewing gum, smoking, and eating after 7 p.m.
- Lactose intolerance is common after abdominal surgery. Patients should eliminate dairy if they experience gas, abdominal bloating, or diarrhea.
- Patients with ileostomies can be deficient in vitamin B12, iron, magnesium, fat, folic acid, sodium, and potassium due to altered absorption. They should be encouraged to consume foods high in sodium (e.g., canned vegetables, broth, and tomatoes) and potassium (e.g., bananas, potatoes, and spinach). Supplementation may be needed.
- Foods that increase the risk of gas include beans, beer, brussel sprouts, carbonated beverages, peas, onions, cauliflower, cabbage, asparagus, and broccoli.
- Foods that thicken stool include bananas, rice, peanut butter, potatoes, tapioca, applesauce, cheese, and pasta.
- Foods that thin stools include grape juice, prune juice, spicy foods, fried foods, and high-sugar foods.
- Foods that increase odor include asparagus, dried beans, peas, onions, fish, garlic, eggs, alcohol, and broccoli.
- Foods that reduce odor include cranberry juice, yogurt, parsley, and buttermilk.
Table 2
Ostomy-Related Patient Education
 References
References
American Cancer Society. (2019a). Caring for a colostomy. https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/surgery/ostomies/colostomy/management.html
American Cancer Society. (2019b). Types of colostomies and pouching systems. https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/surgery/ostomies/colostomy/types-of-colostomies.html
American Cancer Society. (2019c). What is a colostomy? https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/surgery/ostomies/colostomy/what-is-colostomy.html
American Cancer Society. (2020a). Colorectal cancer risk factors. https://www.cancer.org/cancer/colon-rectal-cancer/causes-risks-prevention/risk-factors.html
American Cancer Society. (2020b). Colorectal cancer signs and symptoms. https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/signs-and-symptoms.html
American Society of Colon & Rectal Surgeons. (n.d.). Ostomy expanded information. Retrieved November 20, 2021, from https://fascrs.org/patients/diseases-and-conditions/a-z/ostomy-expanded-version
Azzouz, L. L., & Sharma, S. (2021). Physiology, large intestine. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK507857/
Berti-Hearn, L., & Elliott, B. (2019a). Colostomy care: A guide for home care clinicians. Home Healthcare Now, 37(2), 68-78. https://doi.org/10.1097/NHH.0000000000000735
Berti-Hearn, L., & Elliott, B. (2019b). Ileostomy care: A guide for home care clinicians. Home Healthcare Now, 37(3), 136-144. https://doi.org/10.1097/NHH.0000000000000776
BruceBlaus. (2014). Colostomy types [Image]. https://commons.wikimedia.org/wiki/File:Blausen_0247_Colostomy.png
BruceBlaus. (2017a). Ileostomy [Image]. https://commons.wikimedia.org/wiki/File:Ileostomy.png
BruceBlaus. (2017b). Ostomy emptying bag [Image]. https://commons.wikimedia.org/wiki/File:Ostomy_Emptying_Bag.png
BruceBlaus. (2017c). Ostomy (putting bag on) [Image]. https://commons.wikimedia.org/wiki/File:Ostomy_(Putting_Bag_On).png
Cancer Research UK. (2014a). Diagram showing a colostomy with a bag [Image]. https://commons.wikimedia.org/wiki/File:Diagram_showing_a_colostomy_with_a_bag_CRUK_061.svg
Cancer Research UK. (2014a). Diagram showing the part of the bowel removed with a transverse colectomy [Image]. https://commons.wikimedia.org/wiki/File:Diagram_showing_the_part_of_the_bowel_removed_with_a_transverse_colectomy_CRUK_319.svg
Centers for Disease Control and Prevention. (2021). What are the symptoms of colorectal cancer? https://www.cdc.gov/cancer/colorectal/basic_info/symptoms.htm
Cheng, Z., Dong, S., Bi, D., Wang, Y., Dai, Y., & Zhang, X. (2021). Early versus late preventive ileostomy closure following colorectal surgery: Systematic review and meta-analysis with trial sequential analysis of randomized controlled trials. Diseases of the Colon & Rectum, 64(1), 128-137. https://doi.org/10.1097/DCR.0000000000001839
Fight Colorectal Cancer. (n.d.). Colorectal cancer surgery. Retrieved November 20, 2021, from https://fightcolorectalcancer.org/surgery/
Fish, E. M., & Burns, B. (2021). Physiology, small bowel. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK532263/
Francone, T. D. (2021). Overview of surgical ostomy for fecal diversion. UpToDate. Retrieved November 16, 2021, from https://www.uptodate.com/contents/overview-of-surgical-ostomy-for-fecal-diversion
Hendren, S., Hammond, K., Glasgow, S. C., Perry, W. B., Buie, W. D., Steele, S. R., Rafferty, J., & The American Society of Colon and Rectal Surgeons. (2015). Clinical practice guidelines for ostomy surgery. Diseases of the Colon & Rectum, 58, 375-387. https://doi.org/10.1097/DCR.0000000000000347
Hinkle, J. L., & Cheever, K. H. (2018). Textbook of medical-surgical nursing (14th ed.). Wolters Kluwer.
Kozell, K., Frecea, M., & Thomas, J. T. (2014). Preoperative ostomy education and stoma site marking. Journal of Wound, Ostomy & Continence Nursing, 41(3), 206-207. https://doi.org/10.1097/WON.0000000000000031
Landmann, R. G., & Cashman, A. L. (2021). Ileostomy or colostomy care and complications. UpToDate. Retrieved November 16, 2021, https://www.uptodate.com/contents/ileostomy-or-colostomy-care-and-complications
Lfreeman04. (2019). Colon diagram [Image]. https://commons.wikimedia.org/wiki/File:Ds00070_an01934_im00887_divert_s_gif.webp
Lieske, B. (2021). Colon resection. StatPearls [Internet]. https://www.statpearls.com/articlelibrary/viewarticle/19727/
Maria, A., & Lieske, B. (2021). Colostomy care. StatPearls [Internet]. https://www.statpearls.com/articlelibrary/viewarticle/19743/
MarleneBu. (2018). Ileoanal reservoir [Image]. https://commons.wikimedia.org/wiki/File:Ileoanal_Reservoir.jpg
Mayo Clinic. (2020). Ostomy: Adapting to life after colostomy, ileostomy, or urostomy. https://www.mayoclinic.org/diseases-conditions/colon-cancer/in-depth/ostomy/art-20045825
Mayo Clinic. (2021). Intestinal obstruction. https://www.mayoclinic.org/diseases-conditions/intestinal-obstruction/symptoms-causes/syc-20351460
Migaly, J., Bafford, A. C., Francone, T. D., Gaertner, W. B., Eskicioglu, C., Bordeianou, L., Feingold, D. L., & Steele, S. R. (2018). The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the use of bowel preparation in elective colon and rectal surgery. Diseases of the Colon & Rectum, 62(1), 3-8. https://doi.org/10.1097/DCR.0000000000001238
Mulita, F. (2021). Intestinal stoma. StatPearls [Internet]. https://www.statpearls.com/ArticleLibrary/viewarticle/122144
National Cancer Institute. (2021). Colorectal cancer prevention. https://www.cancer.gov/types/colorectal/patient/colorectal-prevention-pdq
National Institute of Diabetes and Digestive and Kidney Diseases. (2014). Digestive diseases statistics for the United States. US Department of Health and Human Services. https://www.niddk.nih.gov/health-information/health-statistics/digestive-diseases
National Institute of Diabetes and Digestive and Kidney Diseases. (2017a). Definition & facts for Crohn's disease. US Department of Health and Human Services. https://www.niddk.nih.gov/health-information/digestive-diseases/crohns-disease/definition-facts
National Institute of Diabetes and Digestive and Kidney Diseases. (2017b). Symptoms & causes of Crohn's disease. US Department of Health and Human Services. https://www.niddk.nih.gov/health-information/digestive-diseases/crohns-disease/symptoms-causes
National Institute of Diabetes and Digestive and Kidney Diseases. (2017c). Treatment Crohn's disease. US Department of Health and Human Services. https://www.niddk.nih.gov/health-information/digestive-diseases/crohns-disease/treatment
National Institute of Diabetes and Digestive and Kidney Diseases. (2017d). Your digestive system & how it works. US Department of Health and Human Services. https://www.niddk.nih.gov/health-information/digestive-diseases/digestive-system-how-it-works
National Institute of Diabetes and Digestive and Kidney Diseases. (2020a). Definition & facts of ulcerative colitis. US Department of Health and Human Services. https://www.niddk.nih.gov/health-information/digestive-diseases/ulcerative-colitis/definition-facts
National Institute of Diabetes and Digestive and Kidney Diseases. (2020b). Symptoms & causes of ulcerative colitis. US Department of Health and Human Services. https://www.niddk.nih.gov/health-information/digestive-diseases/ulcerative-colitis/symptoms-causes
National Institute of Diabetes and Digestive and Kidney Diseases. (2020c). Treatment for ulcerative colitis. US Department of Health and Human Services. https://www.niddk.nih.gov/health-information/digestive-diseases/ulcerative-colitis/treatment
National Institute of Diabetes and Digestive and Kidney Diseases. (2021a). Definition & facts for diverticular disease. US Department of Health and Human Services. https://www.niddk.nih.gov/health-information/digestive-diseases/diverticulosis-diverticulitis/definition-facts
National Institute of Diabetes and Digestive and Kidney Diseases. (2021b). Definitions and facts for ostomy surgery of the bowel. US Department of Health and Human Services. https://www.niddk.nih.gov/health-information/digestive-diseases/ostomy-surgery-bowel/definition-facts
National Institute of Diabetes and Digestive and Kidney Diseases. (2021c). Symptoms and causes of diverticular disease. US Department of Health and Human Services. https://www.niddk.nih.gov/health-information/digestive-diseases/diverticulosis-diverticulitis/symptoms-causes
National Institute of Diabetes and Digestive and Kidney Diseases. (2021d). Types of ostomy surgery of the bowel. US Department of Health and Human Services. https://www.niddk.nih.gov/health-information/digestive-diseases/ostomy-surgery-bowel/types#ileocolostomy
National Institute of Diabetes and Digestive and Kidney Diseases. (2021e). What to expect before & during ostomy surgery of the bowel. US Department of Health and Human Services. https://www.niddk.nih.gov/health-information/digestive-diseases/ostomy-surgery-bowel/what-to-expect-before-during
Olinsherwood216. (2020). Ostomy skin barriers [Image]. https://commons.wikimedia.org/wiki/File:Ostomy_Skin_Barriers.jpg
OpenStax College. (2013). Components of the digestive system [Image]. https://commons.wikimedia.org/wiki/File:2401_Components_of_the_Digestive_System.jpg
Polsinelli, E. (2015). Ostomy wafer being worn by an ileostomy patient [Image]. https://commons.wikimedia.org/wiki/File:Ostomy_wafer_being_worn_by_an_ileostomy_patient.jpg
Rajaretnam, N., & Lieske, B. (2021). Ileostomy. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK519003/
Registered Nurses' Association of Ontario. (2019). Supporting adults who anticipate or live with an ostomy (2nd ed.). International Affairs & Best Practice Guidelines. https://rnao.ca/sites/rnao-ca/files/Ostomy_Care__Management.pdf
Remedios44. (2010a). Ileostomy001 [Image]. https://commons.wikimedia.org/wiki/File:Ileostomy001.jpg
Remedios44. (2010b). Ileostomy bag [Image]. https://commons.wikimedia.org/wiki/File:Ileostomy_bag.jpg
Rodriguez-Bigas, M. A. (2020). Overview of colon resection. UpToDate. Retrieved November 20, 2021, from https://www.uptodate.com/contents/overview-of-colon-resection
Samir. (2015). Crohn's disease vs. colitis ulcerosa [Image]. https://commons.wikimedia.org/wiki/File:Crohn%27s_Disease_vs_Colitis_ulcerosa.svg
Stelton, S. (2019). CE: Stoma and peristomal skincare: A clinical review. American Journal of Nursing, 119(6), 38-45. https://doi.org/10.1097/01.NAJ.0000559781.86311.64
United Ostomy Association of America. (n.d.-a). Ileostomy facts. Retrieved November 20, 2021, from https://www.ostomy.org/ileostomy/
United Ostomy Association of America. (n.d.-b). What is an ostomy? Retrieved November 20, 2021, from https://www.ostomy.org/what-is-an-ostomy/
United Ostomy Association of America. (2018). Facts about ostomy reversals. https://www.ostomy.org/facts-ostomy-reversals/
United Ostomy Association of America. (2020). New ostomy patient guide. https://www.ostomy.org/wp-content/uploads/2020/10/UOAA-New-Ostomy-Patient-Guide-2020-10.pdf
Wound, Ostomy, and Continence Nurses Society. (n.d.). Make an impact as a WOC nurse. Retrieved November 22, 2021, from https://www.wocn.org/wp-content/uploads/2021/08/WOCN_Nurse_Impact_Infographic_8.30.2021.pdf
Wound, Ostomy, and Continence Nurses Society. (2014). Discharge planning for a patient with a new ostomy. https://cdn.ymaws.com/member.wocn.org/resource/resmgr/document_library/Discharge_Planning_Pt_New_Os.pdf
Wound, Ostomy, and Continence Nurses Society Guideline Development Task Force. (2018). WOCN society clinical guideline: Management of the adult patient with a fecal or urinary ostomy - An executive summary. Wound, Ostomy, and Continence Nursing, 45(1), 50-58. https://doi.org/10.1097/WON.0000000000000396
Wound, Ostomy, and Continence Nurses Society Peristomal Skin Complications Task Force. (2015). Peristomal skin complications: Clinical resource guide. https://cdn.ymaws.com/member.wocn.org/resource/resmgr/document_library/Peristomal_Skin_Complication.pdf
Wound, Ostomy, and Continence Nurses Society Task Force. (2018). Wound, ostomy, and continence nursing: Scope and standards of WOC practice, 2nd edition. Journal of Wound, Ostomy & Continence Nursing, 45(1), 50-58. https://doi.org/10.1097/WON.0000000000000396