About this course:
This course reviews the background, pathophysiology, management, and indications for patients requiring mechanical ventilation (MV), including ventilator modes, settings, complications, consequences, troubleshooting, and safety considerations to optimize patient outcomes.
Course preview
This course reviews the background, pathophysiology, management, and indications for patients requiring mechanical ventilation (MV), including ventilator modes, settings, complications, consequences, troubleshooting, and safety considerations to optimize patient outcomes.
After this activity, learners will be prepared to:
- discuss the background and incidence of MV for critically ill patients.
- describe the pathophysiology of MV in the respiratory system.
- identify indications for MV in critically ill patients.
- describe various ventilator modes, including common initial ventilator settings.
- discuss various mechanisms to troubleshoot ventilator problems.
- explain various complications and consequences of MV and their effects on patient outcomes.
- explore strategies to prevent MV-related complications.
Healthcare providers (HCPs) are responsible for offering high-quality, evidence-based care to optimize patient outcomes. As new treatments emerge, people are living longer, healthier lives. As the US population continues to age, more people are living with chronic health conditions. As more people are managing their complex chronic conditions, increasing numbers of patients are admitted to critical care units (Dirkes & Kozlowski, 2019). Traditionally, HCPs working with critically ill patients have focused on stabilizing immediate, life-threatening cardiopulmonary symptoms. As survival from critical illness has improved, the medical focus has shifted to preventing the sequelae of critical illness. Critically ill patients are often admitted to ICUs so that HCPs can manage physiological responses to illness effectively. MV is frequently used for critically ill patients requiring airway protection or respiratory support. MV is defined as delivering positive pressure to the lungs through a tracheostomy or endotracheal (ET) tube (Hickey et al., 2024; Hyzy & McSparron, 2024). HCPs caring for patients requiring MV must be able to describe the pathophysiology of MV in the respiratory system, identify appropriate indications for MV, and differentiate the various ventilator settings, including their appropriate indications. In addition, HCPs must describe the necessary assessment and management strategies for patients requiring MV, including monitoring for various complications, troubleshooting ventilator problems, and identifying strategies to promote ventilator weaning.
Background
The CDC defines chronic diseases as conditions that last more than 1 year and require ongoing medical attention and/or limit activities of daily living. Chronic disease is the leading cause of death and disability in the US. An estimated six out of 10 American adults have at least one chronic disease, and four out of 10 have two or more chronic diseases. Chronic conditions such as heart disease, cancer, chronic lung disease, diabetes mellitus, Alzheimer's disease, and chronic kidney disease contribute significantly to the $4.5 trillion spent on US health care costs annually. The current life expectancy for adults in the US is 77.5 years (CDC, 2024a, 2024b, 2024c). As the population lives longer with complex chronic conditions, more advanced medical treatments will be necessary. More than 5 million patients are admitted to ICUs in the US annually for intensive or invasive monitoring, including airway, breathing, or circulation support; stabilization of acute or life-threatening medical problems; and comprehensive management of an injury or illness. Although the ICU patient population is heterogeneous, the most common indications for admission include cardiac, respiratory, and neurological conditions. Respiratory failure with ventilator support is among the top five reasons for ICU admissions for adults. In addition, the most common technological support required in an ICU is MV, accounting for 20% to 40% of admissions in the US. Annual critical care costs have increased by 92% between 2000 and 2010[MR1] [SM2] , rising from $56 billion to $108 billion. ICU costs per day were estimated to be $4,300 in 2010, representing a 61% increase since 2000. Although more recent data on critical care costs are not reported in the literature, it is expected that health care expenditures will rise 5.4% annually from 2022 to 2031 (Society of Critical Care Medicine [SCCM], 2024). The number of patients admitted to the ICU for respiratory support is expected to rise further due to the COVID-19 pandemic, with rates of MV among these patients ranging from 29% to 89% across countries (Wunsch, 2020).
With the increase in patients receiving MV, HCPs will see more patients requiring prolonged mechanical ventilation (PMV). The Centers for Medicare and Medicaid Services defines PMV as more than 21 days of MV for at least 6 hours per day. An estimated 300,000 patients in the US require MV each year. Of these patients, between 4% and 13% require PMV, which equates to between 7,250 and 11,400 patients undergoing PMV at any time. Successful ventilator weaning occurs when a patient has 7 consecutive days without the use of ventilatory support. HCPs should frequently assess for weaning readiness to prevent complications associated with MV. PMV is associated with increased length of stay, health care costs, ventilator-associated events (VAEs), morbidity, and mortality. Therefore, the prevention of PMV and VAEs is essential for HCPs to optimize patient outcomes (Jarrett et al., 2016; King Han, 2023; National Healthcare Safety Network [NHSN], 2024; Villalba et al., 2019).
MV is a common cause of ICU admission for patients requiring airway protection or respiratory support. By performing the work of breathing (WOB) and gas exchange, MV can fully or partially replace the functions of spontaneous breathing for patients with respiratory failure. During MV, a predetermined air mixture (i.e., oxygen [O2] and other gases) is forced into the central airways and travels into the alveoli. As a result, the lungs inflate due to increased intra-alveolar pressure. The ventilator stops forcing air into the central airways when a termination signal occurs, usually from increased flow or pressure. Expiration happens passively as the central airway pressure decreases, with air flowing from the higher-pressure alveoli to the lower-pressure central airways (Hyzy & McSparron, 2024; Mora Carpio & Mora, 2023). HCPs caring for patients requiring MV must understand the following ventilator-related concepts (Hinkle et al., 2021; Mora Caprio & Mora, 2023; Respiratory Therapy Zone, 2024):
- Ventilation is the movement and exchange of gases (O2 and carbon dioxide [CO2]) between the lungs and the air. A ventilator forces O2 into the lungs, and CO2 is removed from the body during exhalation. In a patient on MV, the CO2 in the blood can be modified by changing the tidal volume (TV) or respiratory rate.
- Oxygenation of mechanically ventilated patients, which boosts the O2 supply to the lungs, can be achieved by increasing the fraction of inspired oxygen (FiO2) or the positive end-expiratory pressure (PEEP).
- TV is the volume of air moved in and out of the lungs with each respiratory cycle.
- PEEP refers to the positive pressure applied by the ventilator at the end of each respiratory cycle. In mechanically ventilated patients, the pressure will remain greater than the atmospheric pressure to prevent the alveoli from...
...purchase below to continue the course
Indications for MV
A mechanical ventilator is used to decrease the WOB until patients can resume normal breathing on their own. A ventilator ensures that a patient receives adequate O2 to be used by the tissues and removes CO2. Many patients requiring MV can breathe spontaneously, although the effort needed to do so can be exhausting. By partially or fully assisting with respiration, MV preserves a stable airway and allows for respiratory muscle relaxation while a patient recovers from an illness or injury. The most common indication for intubation and MV is acute respiratory failure. Respiratory failure is a condition in which the lungs cannot adequately perform gas exchange, resulting in hypoxia, hypercarbia, and persistent acidosis (decreased pH). MV may also be indicated for patients requiring airway protection to reduce the risk of aspiration (e.g., neurological impairment from drugs, poisons, spinal cord injury, or myasthenia gravis). Following a traumatic injury, patients may require MV depending on the injury's severity and location (e.g., head, neck, and chest). Other indications for MV can include cardiopulmonary arrest, cardiovascular impairment (e.g., strokes, emboli, tumors), and pulmonary impairment (e.g., tumors, infections, COPD, pneumothorax; American Thoracic Society, 2020; Hinkle et al., 2021; Hyzy & McSparron, 2024; Mora Carpio & Mora, 2023; Patel, 2024b). Regardless of the indication, HCPs should consider the need for MV early in the course of illness to prevent emergent intubation.
Pathophysiology
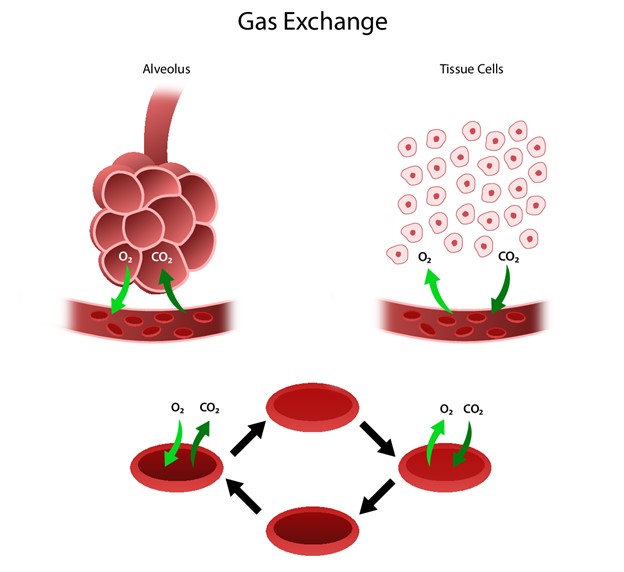
The respiratory system facilitates life-sustaining processes, including O2 transport, respiration, ventilation, and gas exchange. The body's cells rely on the oxidation of carbohydrates, fats, and proteins to produce energy. Without a continuous supply of O2, cells in the brain, heart, and other essential organs cannot survive. O2 is transported to, and CO2 is removed from, the circulating blood through the thin walls of the capillaries. O2 diffuses through capillary walls to the interstitial fluid and eventually to the cells. CO2 diffuses in the opposite direction from the cells to the blood. After these tissue capillary exchanges, blood enters the systemic venous circulation and travels to the pulmonary circulation. The O2 concentration in the alveoli is higher than in the blood. Therefore, O2 diffuses from the alveoli to the blood. Similarly, the blood in the pulmonary artery has a higher concentration of CO2 than the alveoli, so it diffuses from the blood to the alveoli (see Figure 1; Hinkle et al., 2021).
Figure 1
Human Gas Exchange
Ventilation
Ventilation requires movement of the walls of the thoracic cage and the diaphragm. The movements of the thoracic cage and diaphragm alternate by increasing and decreasing the chest's capacity. As the thoracic cavity expands, the pressure inside the thorax is lower than the atmospheric pressure; air flows from a region of higher pressure to lower pressure. When the chest capacity increases, air enters the trachea, passes through the bronchi and bronchioles, and inflates the alveoli in the lungs (inspiration). During expiration, the diaphragm relaxes, and the lungs recoil, decreasing the size of the thoracic cavity. As the pressure in the alveoli increases, air flows from the lungs to the atmosphere. The inspiratory phase of respiration requires active energy, while the expiratory phase is passive (Hinkle et al., 2021; Powers et al., 2023).
In addition to air pressure variances, airway resistance, lung compliance, and lung volumes can also impact ventilation. The size or diameter of the airway, lung volumes, and airflow velocity determine airway resistance. Any process that changes the diameter of the airway will affect airway resistance and the rate of airflow. With increased airway resistance, a more significant respiratory effort will be required to achieve normal ventilation. Compliance allows for the thoracic cavity to expand (change in volume) based on air pressure variances. Alveolar surface tension and the amount of connective tissue and water in the lungs determine lung compliance. Standard compliance allows the lungs and thorax to stretch easily when pressure is applied. Increased compliance occurs when the lungs have lost their elastic recoil and become permanently overextended (e.g., COPD). Decreased compliance occurs when the lungs become stiff, requiring increased energy expenditure to achieve normal ventilation levels. Acute respiratory distress syndrome (ARDS), pulmonary edema, pleural effusion, class 3 obesity (BMI greater than 40), pneumothorax, and pulmonary fibrosis are associated with decreased lung compliance (Hinkle et al., 2021; Powers et al., 2023).
Perfusion
Gas exchange depends on pulmonary diffusion and perfusion. Pulmonary diffusion is how O2 and CO2 are exchanged in the body due to the differences in gas concentrations in the alveoli and capillaries. The alveolar-capillary membrane is ideal for diffusion due to its large, thin surface area, allowing for gas exchange from areas of high concentration to low concentration. Pulmonary perfusion refers to the actual blood flow through the pulmonary vasculature and is determined by pulmonary artery pressure, gravity, and alveolar pressure. Blood is pumped into the lungs by the right ventricle through the pulmonary artery. The pulmonary artery supplies both lungs by dividing into the right and left branches. The normal range for systolic blood pressure in the pulmonary artery is about 20 to 30 mm Hg, and the diastolic pressure is 5 to 15 mm Hg, making pulmonary circulation a low-pressure system. Due to these lower pressures, the pulmonary vasculature can accommodate varying amounts of blood flow. Perfusion is also influenced by alveolar pressure, as pulmonary capillaries lie between adjacent alveoli. Therefore, increased alveolar pressure squeezes the capillaries and impacts perfusion (Hinkle et al., 2021; Powers et al., 2023).
Adequate gas exchange depends on effective ventilation and perfusion, resulting in an adequate ventilation-perfusion (V/Q) ratio. Four types of V/Q states can occur in the lungs: normal V/Q ratio, low V/Q ratio, high V/Q ratio, and absence of ventilation and perfusion. In a healthy lung, the V/Q ratio is 1:1, indicating equal amounts of blood and gas moving through the alveoli. A low V/Q ratio (shunting) happens when perfusion exceeds ventilation. This can result in hypoxia as blood bypasses the alveoli without gas exchange and often occurs due to obstruction of the distal airways (e.g., tumor, atelectasis, pneumonia, mucus plug). A high V/Q ratio (dead space) develops when ventilation exceeds perfusion, resulting in an inadequate blood supply for gas exchange in the alveoli. This may be due to conditions such as pulmonary emboli (PE), pulmonary infarction, or cardiogenic shock. A silent unit is an area of the lung without ventilation or perfusion typically caused by blockages (e.g., pneumothorax, ARDS; Hinkle et al., 2021; Powers et al., 2023).
Neurologic Control of Ventilation
Ventilation control depends on the neuronal network in the brainstem, which controls the activities of the motor neurons that innervate the respiratory muscles. The inspiratory and expiratory centers in the medulla oblongata and pons control the rate and depth of the ventilation. The pneumotaxic center in the upper pons controls the respiration pattern, while the apneustic center in the lower pons stimulates the inspiratory medullary center to promote deep inspiration. In addition, various groups of receptor sites in the brain assist with respiratory control. Central chemoreceptors within the medulla respond to chemical changes in cerebrospinal fluid. An increase or decrease in pH triggers these receptors, resulting in a change in the rate and depth of respiration. Peripheral chemoreceptors within the aorta to the arch and in the carotid arteries respond to changes in PaO2, followed by changes in PaCO2 and pH. Within the lungs, various receptors—including stretch, irritant, and juxtacapillary mechanoreceptors—respond to changes in resistance by altering breathing patterns. Proprioceptors in the muscles and chest wall react to body movements such as range-of-motion exercises, stimulating an increase in ventilation. Finally, baroreceptors in the aorta and carotid bodies respond to changes in arterial blood pressure, causing reflex hypoventilation or hyperventilation (Hinkle et al., 2021; Powers et al., 2023).
Mechanical Ventilation
As previously discussed, normal respiratory function works as a negative pressure system. During inspiration, the diaphragm moves downward, creating negative pressure in the pleural cavity. This negative intrathoracic pressure decreases right atrial pressure, which creates a pulling effect on the inferior vena cava, resulting in increased venous return. Conversely, MV pushes air into the upper airways and alveoli, creating positive pressure in the thoracic cavity. This positive intrathoracic pressure increases right atrial pressure and decreases venous return, reducing preload (i.e., the force stretching the cardiac muscle before constriction). With less blood reaching the right ventricle and, subsequently, the left ventricle, cardiac output decreases. With decreased preload and cardiac output, mean arterial pressure will drop if there is no compensatory increase in systemic vascular resistance. In addition, the positive pressure generated by MV can significantly reduce a patient's WOB. This reduction in WOB allows blood flow to be redistributed to more critical organs, limits CO2 and lactate generation, and improves acidosis. Unfortunately, this also induces respiratory muscle and diaphragmatic weakness, a significant predictor of PMV (see Figure 2; King Han, 2023; Mora Carpio & Mora, 2023).
HCPs who manage patients receiving MV must understand lung pressures and lung compliance. Healthy lung compliance in adults is approximately 100 mL/cm H2O. Therefore, when positive-pressure ventilation delivers 500 milliliters of air to a healthy lung, a 5-cm H2O increase in alveolar pressure will occur. Various respiratory disorders can significantly impact lung compliance. For example, any respiratory disorder that destroys the lung parenchyma (e.g., COPD) can increase lung compliance. Conversely, ARDS, pneumonia, pulmonary fibrosis, and pulmonary edema can cause stiffening of the lungs, resulting in decreased lung compliance. Stiffening can increase the risk of barotrauma during MV, where small increases in volume can lead to significant increases in pressure. HCPs should understand two pressure types when managing a patient receiving MV: peak pressure and plateau pressure. Peak pressure is a reflection of airway resistance and is the maximum pressure when air is pushed into the lungs during inspiration. Plateau pressure is a static pressure achieved at the end of full inspiration during an inspiratory hold, representing alveolar pressure and lung compliance. Normal plateau pressure is below 30 cm H2O; higher pressures increase the likelihood of barotrauma (Mora Carpio & Mora, 2023; Patel, 2024b).
Figure 2
Biology of Ventilation

(Lutz, 2020)
Ventilator Management
Caring for critically ill patients requiring MV is complex and requires HCPs to understand how to prepare for, initiate, and manage ventilator settings. Inappropriate management of ventilator settings can result in poor patient outcomes (Williams & Sharma, 2023). Before the initiation of MV, HCPs must first determine that a patient needs ventilatory support. As discussed above, there are various indications for MV, and determining when to initiate ventilatory support requires an interprofessional health care team. In the past, initiating invasive MV (e.g., intubation with an ET tube) was done late in the course of illness (e.g., respiratory arrest). However, evidence now supports early intubation when indicated. The decision to institute MV should be based on clinical signs and symptoms in conjunction with each patient's wishes for life-sustaining measures (Allen, 2024). Table 1 outlines laboratory and clinical manifestations that indicate the need for MV (Hinkle et al., 2021; Respiratory Therapy Zone, 2024).
Table 1
Laboratory and Clinical Manifestations for Initiation of MV
Laboratory Findings (reference range) | Clinical Manifestations |
PaO2 < 55 mm Hg (80 to 100 mm Hg) | Apnea or bradypnea |
PaCO2 > 50 mm Hg (35 to 45 mm Hg) and pH < 7.32 (7.35 to 7.45) | Respiratory distress with confusion |
VC < 10 mL/kg (65 to 75 mL/kg) | Increased WOB not relieved by other interventions |
NIF < -25 cm H2O (> -60 cm H2O) | Confusion requiring airway protection |
FEV1 < 10 mL/kg (80% of predicted) | Circulatory shock |
Controlled hyperventilation (e.g., a severe head injury) |
(Hinkle et al., 2021; Respiratory Therapy Zone, 2024)
HCPs play an essential role in the identification of patients with respiratory or airway compromise. A proactive approach with early intubation, when indicated, can prevent the need for emergent intubation. For HCPs working in emergency departments, emergent intubation may be necessary in certain circumstances. Specialized training is necessary for emergency HCPs who may have to perform tracheal intubation under stressful conditions. Successful intubation requires a good understanding of various methods of intubation, potentially difficult intubations, medications best suited for airway management, and management of a difficult or failed airway. With a wide range of potential respiratory or airway management scenarios, the need for intubation may be unclear. HCPs must consider several factors when determining whether intubation is appropriate, including each patient's respiratory status, pathologic process and the likelihood of deterioration, teaching received, comorbidities, and available resources (Brown, 2023; Hou & Baez, 2023). If the need for intubation is not immediately apparent, HCPs should consider the following three questions when deciding to intubate versus observe. An affirmative answer to any of these questions confirms the need for intubation in most situations (Brown, 2023).
- Is patency or protection of the airway at risk? First, HCPs must determine whether a patient has a patent airway. Intubation is necessary if the airway is not patent or if a patient has lost their protective airway reflexes. The ability to swallow secretions and phonate clearly are reliable indicators of airway protection. A patient who can phonate clearly and answer questions appropriately demonstrates adequate ventilation and airway protection. In addition, the neurologic complexity of swallowing demonstrates protective airway reflexes. A patient with a decreased level of consciousness, an inability to phonate clearly, pooling secretions, and an inability to swallow requires intubation (Brown, 2023).
- Is oxygenation or ventilation failing? Second, HCPs must determine if the patient can maintain oxygenation and ventilation. Oxygen is essential for cellular respiration and effective organ function. Anaerobic metabolism can maintain skeletal muscle function for a short time. However, neuronal and myocardial tissue will sustain irreversible damage within minutes without an adequate O2 supply. Noninvasive positive-pressure ventilation (NIPPV) can be used in some situations to avoid invasive intubation and will be discussed in more detail. If a patient cannot oxygenate effectively despite supplemental O2, intubation is warranted. HCPs should evaluate patients for early signs of hypoxia (e.g., restlessness, agitation, cyanosis, tachycardia). Clinical signs of worsening hypoxia include confusion, somnolence, and bradycardia. Pulse oximetry is an accurate measure of arterial oxygenation but an unreliable indicator of peripheral perfusion. The removal of CO2 depends on effective lung function (ventilation). HCPs can evaluate ventilation by assessing respirations, mental status, and end-tidal CO2 through capnography (Brown, 2023).
- Is a need for intubation anticipated? HCPs must be able to anticipate conditions requiring intubation when patients currently have a patent airway and adequate oxygenation and ventilation. Considering each patient's current state, underlying disease processes, and the anticipated clinical course will help determine whether preemptive intubation is warranted, regardless of airway patency and adequate oxygenation and ventilation. There is no guideline or algorithm for preemptive intubation. Therefore, HCPs must use their assessment skills and clinical judgment for each patient scenario (Brown, 2023).
Once an intubation decision is made, HCPs should ensure proper ET tube placement. Verification of proper ET tube placement can be done using a combination of clinical and radiological findings (Mora Carpio & Mora, 2023). According to the American College of Emergency Physicians (ACEP, 2022), a video laryngoscope visualizes the ET tube passing through the vocal cords into the trachea, which constitutes firm evidence of correct placement. However, additional objective findings can help confirm its placement. An end-tidal CO2 detector (i.e., continuous waveform capnography, colorimetric, or non-waveform capnography) can be used for patients who have adequate perfusion. Clinical assessment methods—auscultation of the chest and epigastrium, visualization of thoracic movement, fogging in the tube, pulse oximetry, and chest radiography—are not sufficiently reliable sole indicators of accurate placement (ACEP, 2022).
In addition to ensuring proper ET tube placement, cardiovascular support should be established with fluids and vasopressors as indicated. Finally, adequate sedation and analgesia are also essential to reduce restlessness and control pain associated with an ET tube (Mora Carpio & Mora, 2023). Noninvasive and invasive positive-pressure ventilation are complex processes that require interprofessional collaboration. HCPs should check all equipment to ensure proper functioning before patient use (Potchileev et al., 2023).
Noninvasive Ventilation
Not all patients needing breathing support require intubation. Noninvasive positive-pressure ventilation (NIPPV) can be an effective alternative to intubation, especially for patients with acute exacerbations of COPD, early ARDS, and acute cardiogenic pulmonary edema (CPE). For patients presenting with acute respiratory failure due to these conditions, the successful use of NIPPV can prevent intubation and reduce the risk of mortality. However, for patients with severe respiratory distress, high aspiration risk, or airway compromise, NIPPV is not appropriate. NIPPV includes continuous positive airway pressure (CPAP) and bi-level positive airway pressure (BiPAP). CPAP is the noninvasive equivalent of continuous PEEP, while BiPAP combines CPAP and pressure support ventilation (PSV). Both methods deliver positive airway pressure to assist breathing using a noninvasive modality (e.g., nasal mask, face mask, or nasal plugs) rather than an ET tube or tracheostomy. Helmets that deliver NIPPV are available as an alternative for patients who cannot tolerate a tight-fitting mask. The COVID-19 pandemic has increased the use of noninvasive ventilation modes to avoid intubation, including CPAP, BiPAP, and high-frequency ventilation. Both CPAP and BiPAP have lower risks of complications compared to intubation. Some common side effects of CPAP and BiPAP include localized skin damage from the mask, mild abdominal bloating, dry mouth, eye irritation, and sinus pain and congestion. Aspiration is a risk in NIPPV because the airway is not protected. For patients who experience an increased WOB, shock, frequent arrhythmias, or myocardial ischemia, conversion to an ET tube and MV may be necessary (Hou & Baez, 2023; Hyzy & McSparron, 2023; Patel, 2024a).
CPAP
A CPAP machine consists of a pump with a tube attached to a mask covering a patient's mouth and nose. For patients with obstructive sleep apnea, a small mask covering only the nose is often used. CPAP delivers constant positive pressure (throughout inspiration and expiration) utilizing a specific mode on a ventilator or via an independent machine (usually set at a pressure of 5 cm H2O, with a range of 5 to 12 cm H2O). This constant positive pressure opens the upper and lower portions of the airway, preventing the collapse of tissues that occurs during or after exhalation. Thus, CPAP allows for adequate ventilation and oxygenation by preventing the alveoli from collapsing, but a patient must be able to initiate the breath. It is indicated for patients with hypercapnic respiratory failure (PaCO2 greater than 45 mm Hg or pH less than 7.35) due to an acute exacerbation of COPD or acute CPE. Respiratory rate and TV depend solely on the patient's inspiratory effort (Hyzy & McSparron, 2023; Potchileev et al., 2023; Williams & Sharma, 2023).
CPAP provides numerous benefits to patients experiencing respiratory distress, including the following (Hyzy & McSparron, 2023; Mercury Medical, 2014; Pinto & Sharma, 2023).
- With the inspiratory support of CPAP, a patient does not have to work as hard to inhale (decreased WOB), therefore overcoming the auto-PEEP in the lungs. Typically, auto-PEEP is minimal and easily overcome with minimal effort. However, for patients with stiff lungs, auto-PEEP is much harder to overcome.
- Increased inspiratory pressure increases the size and surface area of the alveoli, allowing for increased gas exchange (i.e., increased functional residual capacity).
- CPAP increases the PaO2 in the alveoli, allowing more O2 to diffuse into the bloodstream and decreasing cardiac workload.
- Fluid in the alveolar space can increase WOB, stiffen the lungs, and reduce gas exchange. The pressure from CPAP can force fluid out of the alveolar space and back into the interstitium.
- Patients with COPD have weakened airways that tend to collapse during expiration, causing air trapping. CPAP will splint open the airways during exhalation. The increased resistance can also open nonventilated areas that have collapsed, improving gas exchange.
- CPAP decreases preload and afterload on the heart, resulting in a decreased cardiac workload. This decrease in cardiac workload will lower systolic blood pressure (SBP). Therefore, HCPs should ensure that patients have an SBP of at least 100 mm Hg before initiating CPAP.
BiPAP
BiPAP delivers two levels of positive airway pressure (inspiration and expiration) via a nasal or an oral mask, a nasal pillow, or a mouthpiece with a tight seal and a portable ventilator. This positive airway pressure on inspiration and expiration assists the patient with achieving full TV, resulting in improved ventilation and gas exchange. BiPAP is often used for patients who are hypercapnic. Patients must be able to breathe independently. However, a backup rate can be programmed to ensure a patient receives a set number of breaths per minute (Hinkle et al., 2021; Potchileev et al., 2023).
Due to the two levels of positive airway pressure, BiPAP is the preferred form of NIPPV. An inspiratory positive airway pressure (IPAP) of 8 to 12 cm H2O and expiratory positive airway pressure (EPAP) of 0 to 5 cm H2O are preferred when initiating BiPAP. The TV correlates with the difference between IPAP and EPAP, with an increased TV associated with a larger difference between IPAP and EPAP. BiPAP is generally started at an IPAP/EPAP of 10/5 cm H2O, generating a gradient of at least 5 cm H2O. If additional ventilatory support is needed, both IPAP and EPAP can be increased gradually at 10-minute intervals. IPAP can be increased up to 20 cm H2O, and EPAP can be increased to 10 to 12 cm H2O. Continuous pulse oximetry and end-tidal CO2 monitoring can help determine whether BiPAP has been effective in improving oxygenation and ventilation (Hou & Baez, 2023; Hyzy & Jia, 2024).
Average Volume-Assured Pressure Support
Average volume-assured pressure support (AVAPS) is a relatively new modality of noninvasive ventilation that integrates volume- and pressure-controlled ventilation. In 2009, some ventilators were equipped with AVAPS, a pressure-controlled ventilation setting that replaced IPAP with a targeted TV. Rather than having a fixed IPAP setting, AVAPS allows HCPs to set a range of values for the IPAP (a maximum and minimum). Therefore, the pressure support is no longer fixed, as the IPAP fluctuates within the set range based on the targeted TV (preset). The ventilator uses a feedback loop to increase or decrease the inspiratory pressure to deliver a preset TV. Since the TV will vary with each breath, the ventilator will ensure that the average targeted TV is achieved over 1 minute. AVAPS allows for a smooth transition between inspiratory pressures, preventing ventilator asynchronization. The EPAP for AVAPS is fixed. However, an auto-titration mode regulates EPAP. HCPs will manually set the AVAPS ventilator settings based on the patient's condition and clinical assessment. The target TV is set to 8 mL/kg of ideal weight and adjusted based on the patient's pathology. The maximal IPAP is generally 20 to 25 cm H2O, and the minimum is equal to EPAP plus 4 cm H2O (but no less than 8 cm H2O). AVAPS establishes a respiratory rate of 2 to 3 breaths per minute (BPM) below the resting respiratory rate (Yarrarapu et al., 2023).
IPAP will increase or decrease synchronously with changes in the patient's respiratory effort and lung compliance. Like CPAP and BiPAP, patients receiving AVAPS must be able to initiate breaths independently. Complications associated with AVAPS include nasal congestion, oral and nasal mucosal drying, skin irritation due to the tight facial mask, gastric distention, claustrophobia, and hypotension (in patients with cardiac dysfunction). AVAPS can be beneficial for patients with acute exacerbations of COPD and facilitate successful extubation for patients with ARDS. Even though this mode may appear on some ventilators, BiPAP is usually the preferred method of NIPPV (Yarrarapu et al., 2023).
Invasive Ventilation
Invasive MV assists with ventilation, promotes oxygenation, and decreases the WOB for patients with acute respiratory distress. For patients who meet the criteria described above, a trial of noninvasive ventilation is recommended first. Invasive MV is associated with potentially serious complications that can be avoided using noninvasive ventilation modalities. Patients who fail a trial of noninvasive intubation will then meet the criteria for invasive MV. For patients with a life-threatening clinical presentation of respiratory failure, immediate intubation and MV should be initiated. HCPs should also consider other laboratory and clinical manifestations that warrant MV, as shown in Table 1. However, these laboratory values and clinical manifestations alone cannot determine the need for MV. For example, arterial blood gas (ABG) values may not indicate that MV is necessary, even though the patient is experiencing clinical deterioration. Early intubation in many circumstances can prevent the need for emergent intubation later. An interdisciplinary team of HCPs should evaluate each patient scenario individually while also considering the patient's wishes for life-sustaining interventions. Once the decision is made to intubate, HCPs must determine the appropriate ventilator mode (Allen, 2024; Brown, 2023; Hyzy & McSparron, 2024).
Ventilator modes determine how breaths (inspiratory support) are delivered to patients requiring MV. No universal or initial mode of MV is appropriate for all patients. Some of the most common modes include volume-limited assist control (AC) ventilation, pressure-limited AC, and synchronized intermittent mandatory ventilation (SIMV) with pressure support ventilation (SIMV-PSV). In addition, there is a paucity of evidence indicating that the mode of ventilation affects clinical outcomes. Therefore, ventilator mode selection is generally based on clinician and institutional preferences (Hyzy & Jia, 2024). In addition, health care institutions may use different ventilator manufacturers, each having some variation in modes available. HCPs should be aware that the ventilator simulates four stages of breathing (Hou & Baez, 2023):
- The patient or the ventilator triggers the initiation of inspiration.
- The ventilator provides a breath determined by preset variables (e.g., pressure, volume, and flow rate).
- The ventilator stops inspiration when the preset parameter is achieved (e.g., TV, inspiratory time, or airway pressure).
- The ventilator switches to expiration, and the breath is completed. When the expiratory valve opens, expiration occurs passively through the recoil of the chest wall, lungs, and diaphragm.
Several considerations are involved when selecting the appropriate mode of ventilation for a patient. First, HCPs must determine how the ventilator will deliver the breaths, either through a set volume or a set amount of pressure. When choosing whether the ventilator will deliver volume or pressure, an HCP selects the dependent and independent variables in the lung compliance equation. For example, if the patient is started on volume-controlled ventilation, the ventilator will deliver a fixed volume (independent variable), resulting in a generated pressure dependent on compliance. For patients with poor compliance, the resulting pressure may be high, increasing the risk of barotrauma. Conversely, the ventilator can deliver a fixed pressure (independent variable) during each respiratory cycle if set to pressure-controlled ventilation. In this case, the TV will be dependent on compliance (American Association of Critical Care Nurses [AACN], n.d.; Mora Carpio & Mora, 2023). In either volume- or pressure-controlled ventilation, the HCP will set a respiratory rate, extrinsic or applied PEEP, and FiO2 (Hinkle et al., 2021; Hyzy & Jia, 2024).
Second, HCPs must choose which mode of ventilation to use. Various ventilator modes can assist with all, some, or none of a patient's breaths. HCPs must also select whether the ventilator will deliver breaths even if the patient is not breathing independently. Additionally, HCPs should consider how fast the breath should be delivered (flow), the waveform of flow, and the rate at which breaths will be delivered. A decelerating waveform mimics physiological breaths and is more comfortable for patients. In contrast, square waveforms (when the flow is delivered at full speed throughout inhalation) result in quicker inspiratory times but are often less comfortable. These parameters should be adjusted to maximize patient comfort, attain desired blood gases, and prevent air trapping. These parameters will be discussed in more detail below (AACN, n.d.; Mora Carpio & Mora, 2023).
Volume-Controlled Ventilation
Volume-controlled ventilation, also known as volume-limited or volume-cycled ventilation, delivers a preset air volume with each inspiration (Hinkle et al., 2021). An HCP sets the peak flow rate, flow pattern, and TV. With volume-controlled ventilation, inspiration ends once the inspiratory time has elapsed and the preset volume of air is delivered (Hyzy & Jia, 2024). The ventilator will cycle off, and exhalation will occur passively. The peak inspiratory flow rate will determine the inspiratory time and the inspiratory to expiratory (I:E) ratio. For example, increasing the peak inspiratory flow rate will decrease inspiratory and increase expiratory time, resulting in a reduced I:E ratio. Airway pressures will depend on the ventilator settings and patient-related variables (e.g., compliance and airway resistance). Therefore, larger TV, higher peak flow, poor compliance, or airway resistance will increase airway pressures (peak, plateau, and mean). A significant disadvantage of volume-controlled ventilation is that patients may experience barotrauma due to consistent volume delivery despite increased airway pressures. Volume-controlled ventilation can be delivered utilizing several modes, including controlled mechanical ventilation (CMV), AC, intermittent mandatory ventilation (IMV), and SIMV (Hinkle et al., 2021; Hyzy & Jia, 2024; Mora Carpio & Mora, 2023; Patel, 2024b).
CMV
The CMV mode provides full ventilator support for apneic patients. When using CMV, minute ventilation is determined by the set TV and respiratory rate. Therefore, a patient does not initiate additional breaths as the set minute ventilation meets or exceeds their physiologic need. CMV may be used for patients in a coma, undergoing pharmacologic paralysis, or under heavy sedation (Hyzy & Jia, 2024).
AC
AC is the mode of choice for most ICUs across the US because it is easy to use. With AC mode, the HCP determines the minimal minute ventilation for each patient by setting the TV and respiratory rate. Patients can increase the minute ventilation by triggering additional breaths, with each patient-initiated breath receiving the preset TV. For example, if an HCP sets the TV to 500 milliliters and the respiratory rate to 20 BPM, the patient's minimal minute ventilation is 10 LPM (20 BPM times 0.5 liters per breath). If the patient triggers an additional 5 BPM, the ventilator will deliver 500 milliliters for each breath. Thus, the minute ventilation will increase to 12.5 LPM (25 BPM times 0.5 liters per breath). The volume delivered by the ventilator with each breath will be the same, regardless of compliance, peak, or plateau pressures in the lungs. With AC, each breath can also be time-triggered (at specific intervals) or patient-triggered (when they can breathe independently), making this a more comfortable ventilator setting. HCPs should monitor O2 saturation and ABGs to determine if changes should be made. Advantages of the AC mode include patient comfort, easy corrections for respiratory acidosis/alkalosis, and lower WOB. However, pressure is not directly managed with volume-controlled AC and can increase barotrauma risk (Hyzy & Jia, 2024; Mora-Carpio & Mora, 2023; Patel, 2024b).
IMV
Like AC, with the IMV mode, an HCP determines the minimal minute ventilation by setting the TV and respiratory rate. Patients can increase the minute ventilation, but the mechanism differs from AC. With IMV, patients increase minute ventilation by spontaneous breathing rather than patient-initiated ventilator breaths. Using the same example as above, if an HCP sets the TV to 500 milliliters and the respiratory rate to 20 BPM, the minimal minute ventilation is 10 LPM (20 BPM times 0.5 liters per breath). If the patient adds 5 additional spontaneous breaths, the TV for each additional breath will be determined independently by the patient. Therefore, the minute ventilation will be greater than 10 LPM, depending on the size of the TV for each spontaneous breath. IMV allows patients to use their muscles for respiration, preventing muscle atrophy. In addition, IMV lowers mean airway pressure (mPaw), decreasing the risk of barotrauma. There is an increased risk of patients "fighting the ventilator," which occurs when they attempt to exhale while the ventilator delivers a breath (Hinkle et al., 2021; Hyzy & Jia, 2024; Patel, 2024b).
SIMV
SIMV is a variation of IMV in which HCPs can set the ventilator to synchronize ventilator breaths with a patient's inspiratory effort. Uniquely, SIMV and IMV can be used to titrate the level of ventilatory support needed by patients. SIMV allows for a wide range of ventilatory support from full (setting the respiratory rate high enough that the patient does not breathe over the ventilator) to none (setting the respiratory rate to 0). As with many modes of ventilation, hemodynamic consequences of positive-pressure ventilation can develop. Therefore, a lower level of support provided by SIMV reduces the likelihood of hemodynamic changes. SIMV is a frequently used mode of ventilation and has advantages over some of the other commonly used modes. SIMV offers better patient-ventilator synchrony, enhanced preservation of respiratory muscle function, lower mPaws, and greater control over the level of support. With SIMV, the ventilator senses the patient's breathing efforts. Therefore, the ventilator will not initiate a breath in opposition, reducing "fighting the ventilator." As the patient increases their spontaneous breaths, the WOB becomes more patient-focused. However, compared to AC, independent breaths by the patient will not necessarily receive a full TV or pressure support. Instead, with each breath, the TV pulled by the patient will depend solely on lung compliance and patient effort. For some patients, this could increase WOB and muscle fatigue, making SIMV a poor choice. For patients receiving SIMV, HCPs should monitor respiratory rate, minute ventilation, spontaneous and ventilator-generated TV, FiO2, and ABG levels (Hinkle et al., 2021; Hyzy & Jia, 2024; Mora Carpio & Mora, 2023).
Pressure-Controlled Ventilation
Pressure-controlled ventilation, also known as pressure-limited or pressure-cycled ventilation, delivers airflow until a preset pressure is reached and cycles off and exhalation occurs. HCPs must set the desired inspiratory pressure level and I:E ratio. TV will vary during pressure-controlled ventilation, depending on inspiratory pressure, compliance, airway resistance, and tubing resistance. However, peak airway pressure will remain constant, equaling the sum of the set inspiratory pressure level and applied PEEP. For example, a set inspiratory pressure level of 20 cm H2O and an applied PEEP of 10 cm H2O will result in a peak airway pressure of 30 cm H2O. The limitation of pressure-controlled ventilation is that the volume of air delivered can vary based on airway resistance and compliance, thereby providing inconsistent TV and potentially compromising ventilation (Hinkle et al., 2021; Hyzy & Jia, 2024). Pressure-controlled ventilation can be delivered using the same modes as volume-controlled ventilation (Hyzy & Jia, 2024; Mora Carpio & Mora, 2023; Patel, 2024b):
- With pressure-controlled CMV, also known as pressure control ventilation, the minute ventilation is determined entirely by the set inspiratory pressure level and respiratory rate. Therefore, the patient does not initiate any additional breaths.
- The minute ventilation is determined by the set inspiratory pressure level and respiratory rate with pressure-controlled AC. Like volume-controlled AC, the patient can increase the minute ventilation by initiating additional ventilator-assisted, pressure-controlled breaths.
- With pressure-controlled IMV or SIMV, the set inspiratory pressure level and respiratory rate determine the minimum minute ventilation. With these modes, the patient can increase the minute ventilation by initiating spontaneous breaths.
PSV
PSV is a pressure-controlled and flow-cycled ventilator mode that relies entirely on patient-triggered breaths, as there is no set respiratory rate. With PSV, HCPs will set the inspiratory pressure support level, applied PEEP, and FiO2. TV, respiratory rate, and minute ventilation depend on numerous factors, including ventilator settings, compliance, and patient sedation level. For example, a high inspiratory pressure support level will result in large TVs and a low respiratory rate. With PSV, the WOB is inversely proportional to the pressure support level as long as the support level is sufficient to meet the patient's needs. Therefore, greater pressure support decreases WOB. Similarly, increasing the inspiratory flow rate shortens the time needed to achieve maximal airway pressure, decreasing WOB. As the patient's strength increases, the pressure support is gradually reduced. HCPs must closely monitor each patient's respiratory rate and TV on initiation of PSV with pressure support adjustments to avoid tachypnea or large TVs (Hinkle et al., 2021; Hyzy & Jia, 2024).
PSV gives patients greater control over inspiratory flow and respiratory rates, making it suitable for MV weaning. PSV is frequently combined with SIMV, with the ventilator delivering the set respiratory rate using SIMV and the patient-initiated breaths via PSV. PSV assists patients in overcoming the resistance of the ET tube and ventilator circuit for patient-initiated breaths. The level of PSV needed depends on the resistance of the ET tube, with smaller ET tubes (e.g., below 7 millimeters) requiring higher pressure support levels (e.g., 10 cm H2O or higher). PSV is poorly suited for patients who need full or nearly full ventilatory support and those with increased airway resistance (e.g., COPD or asthma exacerbation) due to the following characteristics (Hyzy & Jia, 2024; Mora-Carpio & Mora, 2023; Patel, 2024b):
- With PSV, patients must initiate each breath. Therefore, apnea can occur if their respiratory drive is depressed due to critical illness, sedatives, or hypocapnia due to excessive ventilation.
- Since TV and respiratory rate are variable, adequate minute ventilation cannot be guaranteed.
- If PSV is used for full ventilatory support, ventilator asynchrony can occur.
- When PSV is used for full ventilatory support, high levels of pressure support (e.g., greater than 20 cm H2O) are required to prevent alveolar collapse. However, high levels of pressure support are more uncomfortable for patients and can increase the likelihood of cyclic atelectasis and ventilator-associated lung injury (VLI).
- For patients with increased airway resistance, minute ventilation is likely to be insufficient. High airway resistance can decrease airflow, causing inspiration to terminate before an optimal TV is delivered. PSV can also increase WOB and worsen respiratory muscle fatigue because it does not reduce auto-PEEP.
Automatic tube compensation is a type of PSV that applies sufficient positive pressure to overcome the WOB imposed by the ET tube, which can vary from breath to breath. Automatic tube compensation can be combined with other ventilator modes and is often used for spontaneous breathing trials (SBTs). Patients undergoing SBTs utilizing automatic tube compensation are more likely to tolerate the trial than those with CPAP alone (Hyzy & Jia, 2024).
Pressure-Regulated Volume Control
Pressure-regulated volume control (PRVC) is a form of volume- and pressure-controlled ventilation. With PRVC, an HCP presets the TV, and the applied airway pressure changes to reach the target TV. The difference in pressure required by the previous breath to attain the set TV determines the applied inspiratory pressure for the next breath. With PRVC, inspiratory flow is variable and changes with patient effort and lung mechanics. The variability of inspiratory flow is often more comfortable for patients. The PRVC mode may not be universally available and varies with the ventilator manufacturer (Hyzy & Jia, 2024). With PRVC, HCPs should set the inspiratory time instead of flow, with a target I:E ratio of 1:2 to 1:3. See Table 2 for additional volume-controlled ventilator settings that would also be used with PRVC (Hyzy & Jia, 2024; Hyzy & McSparron, 2024).
Airway Pressure Release Ventilation
Airway pressure release ventilation (APRV) is a time-triggered, pressure-controlled, time-cycled ventilator mode that allows for unrestricted, spontaneous breathing throughout the ventilatory cycle. With APRV, breaths may be initiated spontaneously or by the ventilator. During this type of ventilation, a high CPAP is delivered for a long duration and then falls to a lower pressure for a shorter duration. The transition from high to low pressure causes the lungs to deflate and CO2 to be eliminated. As the pressure transitions from low to high, the lungs inflate. The difference between the high and low pressure with APRV is the driving pressure, with larger differences associated with greater inflation and deflation. The driving pressure and compliance will determine the TV with APRV (Hinkle et al., 2021; Hyzy & Jia, 2024; Mora Carpio & Mora, 2023).
In addition, the time associated with the high- and low-pressure components determines the frequency of inflation-deflation cycles. Although there are no universally accepted indications, APRV has been most frequently used for patients with ARDS. For patients with ARDS, the use of APRV has been associated with improved hemodynamics (i.e., oxygenation), compliance, and alveolar recruitment, decreased plateau pressure, more ventilator-free days, and decreased ICU stay. APRV is not recommended for patients with COPD or a high ventilatory requirement because hyperinflation, high alveolar pressure, and barotrauma can occur. The APRV mode may not be available, depending on the manufacturer of the ventilator (Hyzy & Jia, 2024; Mora-Carpio & Mora, 2023).
High-Frequency Oscillatory Ventilation
High-frequency oscillatory ventilation (HFOV) delivers very high respiratory rates (i.e., 180 to 900 BPM) accompanied by constant high airway pressures and low TVs. This high respiratory rate delivers small pulses of oxygen-enriched air into the center of the airways, allowing alveolar air to exit along the margins of the airways while eliminating the inflate-deflate cycle with other modes of MV. This ventilation mode is often used for patients with atelectasis or ARDS to facilitate alveolar opening while protecting the lungs from injury. In addition, HFOV is often used as rescue therapy when other modes of MV fail to improve oxygenation. This ventilation mode has been used across the spectrum of age ranges, from neonates with respiratory distress syndrome to adults with acute lung injury (Hinkle et al., 2021; Hyzy, 2023b; Meyers et al., 2019).
The benefit of HFOV is that the rapid, small pulses of air deliver very high levels of continuous PEEP. Although there are no specific contraindications, HFOV is less effective in disease processes with increased airway resistance, which can cause air trapping and hyperinflation. The utilization of HFOV in these situations can increase the risk of developing a pneumothorax or barotrauma. In addition, patients receiving HFOV require paralytics, sedation, and pain medication, making a neurologic assessment challenging. Therefore, HCPs must closely monitor all patients using HFOV. In addition to clinical appearance and oxygenation levels, ABG levels should be obtained within 15 to 30 minutes of initiation of HFOV and at 30- to 60-minute intervals until stabilization occurs, followed by 6-hour intervals (Hinkle et al., 2021; Hyzy, 2023b; Meyers et al., 2019).
The primary goals of HFOV are to improve clinical outcomes and prevent lung injuries. With active inspiratory and expiratory phases, HFOV produces small TVs, usually equal to or less than dead space. In addition, reasonable oxygenation is delivered with this mode to limit oxygen toxicity. Permissive hypercapnia (allowing PaCO2 to rise while maintaining a pH between 7.25 and 7.3) is used to provide ventilatory support and maintain normal cellular function. These strategies minimize the risk of VLI and secondary chronic lung disease and improve V/Q mismatch while maintaining cardiac output. mPaw and FiO2 are the primary variables used to achieve optimal oxygenation. To introduce mPaw, HCPs should start at 3 to 5 cm H2O above the corresponding mPaw used during conventional ventilation. Normal mPaw ranges from 25 to 30 cm H2O, with a maximum of 45 to 60 cm H2O. The mPaw should not be reduced during the first 24 hours to allow adequate time for alveolar recruitment. The FiO2 is titrated by a blend attached to the oscillator. If the PaO2 is high, the FiO2 and mPaw should be decreased; if the PaO2 is low, the FiO2 and mPaw should be increased (Hyzy, 2023b; Meyers et al., 2019).
The primary variables that impact ventilation include TV, chest wiggle, and frequency. Amplitude can be adjusted by the power control on the ventilator, which regulates the amount of piston displacement; the degree of deflection of the piston (amplitude) determines the TV. As the amplitude increases, the pressure gradient and TV delivered also increase. Therefore, if a patient has low lung compliance, the piston must work against greater pressure, resulting in less change in TV. Similarly, if a patient is underventilated (i.e., their PaCO2 level is high), the amplitude should be increased to blow off more PaCO2. Conversely, PaCO2 will be low during overventilation, and the amplitude should be decreased to increase the PaCO2. HCPs should obtain an ABG when a patient is on conventional MV and add 20 to the PaCO2 level to set the amplitude of HFOV. The power setting should be determined by observing the chest wiggle factor, with chest wiggle visible from the clavicles to the eighth or ninth rib. Increasing amplitude will generate an increase in chest wiggle. Therefore, the HCP should monitor chest wiggle closely throughout HFOV, especially after position changes, for diminished, absent (i.e., ET tube disconnection or obstruction, bronchospasm, or decreased pulmonary compliance), or unilateral wiggle (i.e., ET tube displacement or pneumothorax). With HFOV, frequency is derived from hertz (Hz), controlling the time allowed for the piston to move forward and backward. The range of HFOV is 3 to 15 Hz, with typical initial settings of 5 to 6 Hz; each Hz is 60 BPM. Reducing the frequency can cause greater volume displacement, which increases the TV and minute ventilation. To decrease the PaCO2, the frequency should be reduced or amplitude increased. Conversely, if a patient is overventilated, the frequency should be increased (or amplitude decreased) to boost PaCO2 (Hyzy, 2023b; Meyers et al., 2019).
Initial Ventilator Settings
Once the decision is made to intubate a patient and begin MV, HCPs must know how to input the initial settings properly. Each mechanical ventilator is different, with some manufacturers having additional modes available for use. Therefore, HCPs should review the manufacturer's guidelines and institutional policies when determining mode selection and initial settings. Almost all ventilators can be set to four basic modes: CMV, AC, SIMV, and PSV (Respiratory Therapy Zone, 2024). There is no universal initial mode of MV. However, commonly used initial modes include AC (either volume-controlled or pressure-controlled) or SIMV. PSV is not widely used initially but is more frequently employed for ventilator weaning. As initial ventilation modes, CMV (volume-controlled or pressure-controlled), IMV, and APRV are not typically used. Regardless of the initial mode chosen, HCPs will often change modes or settings when a patient demonstrates intolerance or responds poorly to the selected mode (Hyzy & McSparron, 2024).
HCPs should be aware of factors that influence the choice of ventilation mode (Hyzy & McSparron, 2024):
- Level of support needed: Support depends on a patient's ventilatory needs. AC (volume-controlled or pressure-controlled) provides the most support while resting respiratory muscles and minimizing muscle atrophy. PSV provides the least amount of support and increases WOB.
- Reason for MV: The reason for MV can help HCPs determine the most appropriate mode. Patients with ARDS usually require low TV, delivered by AC (volume-controlled or pressure-controlled). If short-term airway protection is needed, SIMV or PSV might be more appropriate.
- Presence of airflow limitation: Volume-controlled ventilation modes (e.g., volume-controlled AC) are commonly used for patients with airflow limitations (e.g., COPD, acute asthma). Pressure-controlled modes, including APRV, are generally avoided in these patients.
- Presence of an air leak: For patients with a prolonged air leak (e.g., pneumothorax or lung surgery), pressure-controlled ventilation, SIMV-PSV, or PSV alone is preferred. These modes limit additional barotrauma and worsening of the air leak.
- Paralysis: AC (volume-controlled or pressure-controlled) is typically used for patients undergoing heavy sedation or paralysis, although CMV can also be used. PSV is not recommended because patients cannot initiate spontaneous breaths.
The AC mode (volume-controlled) is selected for most patients without parenchymal (interstitial) lung disease or risk factors for ARDS. HCPs must frequently assess each patient's response to the initial settings and adjust them as needed. Once a patient is paralyzed or sedated, their HCP should measure baseline lung compliance, airway resistance, and intrinsic PEEP immediately following the initiation of MV. The level of sedation and analgesia should be frequently monitored for patient comfort. In addition, HCPs should monitor for ventilator problems or complications. For some patients, SIMV with PSV will be chosen as an initial mode of ventilation. With SIMV-PSV, most ventilator settings will be similar to volume-controlled AC with the addition of pressure support for spontaneous breaths taken by the patient above the set rate. Pressure support can be increased or decreased as needed to achieve the desired values and patient comfort. See Table 2 for the initial ventilator settings for volume-controlled AC mode and SIMV-PSV (Hyzy & McSparron, 2024).
For patients in which a pressure-controlled AC mode is chosen, HCPs will set the inspiratory pressure level to target an approximate TV and the inspiratory time to deliver an I:E ratio of 1:2 to 1:3 (typically 1 second). The ventilator rate, applied PEEP, FiO2, and trigger sensitivity will be similar to volume-controlled AC. Initial inspiratory pressures will vary depending on lung compliance, airway resistance, and tubing resistance. However, targeted TVs may be reached with inspiratory pressures between 12 and 25 cm H2O. Peak airway pressures should be monitored due to the increased risk of barotrauma. Higher inspiratory pressures combined with the applied PEEP will increase the peak airway pressures. See Table 2 for the initial ventilator settings for pressure-controlled AC. For patients receiving PSV alone, the pressure support level may be increased up to 20 cm H2O. The pressure is titrated until the patient's respiratory rate is below 30 BPM and the target TV is reached (e.g., 4 to 8 mL/kg predicted body weight [PBW]). Other ventilator parameters—FiO2, applied PEEP, inspiratory flow rate, and trigger sensitivity—are similar to volume-controlled AC mode, as shown in Table 2 (Hyzy & McSparron, 2024).
Table 2
Initial Ventilator Settings for Common Modes of MV
Setting | Volume-Controlled AC Ventilation | SIMV-PSV | Pressure-Controlled AC Ventilation | Considerations |
TV | 6 mL/kg PBW | 6 mL/kg PBW | Inspiratory pressure set to target the desired TV (see below) | TV range is 6 to 8 mL/kg PBW for non-ARDS patients and 4 to 8 mL/kg PBW for ARDS patients. |
Ventilator rate | 12 to 16 BPM | 12 to 16 BPM | 12 to 16 BPM | Rates of ≤ 35 BPM may be necessary for ARDS patients to ensure a low TV. |
PEEP | 5 to 10 cm H2O | 5 to 10 cm H2O | 5 to 10 cm H2O | Lower levels of PEEP can be used for patients with an air leak or who do not have ARDS (e.g., 3 to 5 cm H2O). For hypoxemia from severe ARDS, higher levels may be needed. |
FiO2 | FiO2 to target O2 saturation of 90% to 96% | FiO2 to target O2 saturation of 90% to 96% | FiO2 to target O2 saturation of 90% to 96% | A lower threshold (PaO2 of 55 mm Hg and O2 saturation of 88%) may be appropriate for a patient who is challenging to oxygenate or has hypercapnic hypoxemic respiratory failure. Higher limits may be necessary for carbon monoxide toxicity, cluster headers, sickle cell crisis, pneumothorax, pregnancy, and air embolism. |
Inspiratory flow | 40 to 60 LPM, ramp pattern (decelerating flow waveform) Target an I:E ratio of 1:2 to 1:3. | 40 to 60 LPM, ramp pattern Target an I:E ratio of 1:2 to 1:3. | 40 to 60 LPM, ramp pattern Target an I:E ratio of 1:2 to 1:3. | For patients with airflow obstruction, higher rates up to 75 LPM are appropriate to increase the I:E ratio. |
Trigger sensitivity | 2 LPM (flow-triggered) -1 to -2 cm H2O (pressure-triggered) | 2 LPM (flow-triggered) -1 to -2 cm H2O (pressure-triggered) | 2 LPM (flow-triggered) -1 to -2 cm H2O (pressure-triggered) | If auto-PEEP is suspected, pressure triggering should not be used. |
PSV level | N/A | 5 to 10 cm H2O | N/A | PSV may be adjusted to target the set TV for unsupported breaths. |
Inspiratory pressure | N/A | N/A | Variable (typically between 12 and 25 cm H2O) | The initial inspiratory pressure varies depending on lung compliance, airway resistance, and tubing resistance. |
(Hyzy & McSparron, 2024)
HCPs should be aware of some additional considerations for each ventilator parameter discussed in Table 2 (Hyzy & McSparron, 2024):
- TV: Large TVs (i.e., above 10 mL/kg) can increase the risk of barotrauma and VLI. Studies have shown that low TV decreases mortality in patients with ARDS. An initial TV of 6 mL/kg has been consistently used for patients requiring airway protection, those with respiratory failure, and patients requiring MV for surgery. In numerous studies, patients with lower TVs were less likely to progress to ARDS, develop pneumonia, experience postoperative pulmonary complications, or die. Once the initial TV is set, incremental increases or decreases can achieve the desired pH and PaCO2. HCPs should monitor for auto-PEEP and high airway pressures. Excessively high TVs can increase the risk of barotrauma, auto-PEEP, hyperventilation, and respiratory alkalosis. In contrast, excessively low TVs can lead to atelectasis, hypoventilation, and respiratory acidosis (Hyzy & McSparron, 2024).
- Ventilation rate: A set ventilator rate is required for most volume- and pressure-controlled modes, except for PSV. When a patient breathes above the set ventilation rate, the native respiratory rate and actual TV delivered will determine the true minute ventilation. For patients receiving AC modes, the rate is set to approximately 4 BPM below the native rate. For SIMV, the rate is set to ensure the ventilator delivers at least 80% of the minute ventilation. The ventilator rate can be incrementally increased or decreased to achieve the desired minute ventilation, pH, and PaCO2. Excessively high rates can lead to auto-PEEP and respiratory alkalosis, while excessively low rates can lead to respiratory acidosis (Hyzy & McSparron, 2024).
- PEEP: PEEP is generally used to mitigate end-expiratory alveolar collapse. For patients with ARDS undergoing low TV ventilation, PEEP can be increased from 5 to 24 cm H2O. For non-ARDS patients, excessively high levels of applied PEEP can reduce preload (decreasing cardiac output), elevate plateau pressure (increasing the risk of barotrauma), and impair cerebral venous outflow (increasing intracranial pressure). Hypoxemia and atelectasis can occur from excessively low levels of PEEP (Hyzy & McSparron, 2024).
- FiO2: Supplemental O2 can have adverse consequences, such as absorption atelectasis, accentuation of hypercapnia, airway injury, and parenchymal injury. HCPs should consider using the lowest possible FiO2 necessary to meet oxygenation goals (typically 90% to 96% O2 saturation). Several studies of critically ill patients concluded that hyperoxia (excessive supplemental O2 that increases PaO2 beyond the normal range) should be avoided. However, there was no consistency among the studies in determining what constitutes hyperoxia (Hyzy & McSparron, 2024).
- Flow rate and pattern: A peak inspiratory flow rate is preset for volume-controlled ventilation, with sufficient flow rates decreasing WOB. When a patient's inspiratory flow rate is insufficient, they may experience dyspnea from increased WOB. For patients with obstructive airway disease, higher peak flow rates are needed, which shortens the inspiratory time and increases the expiratory time (i.e., decreases the I:E ratio). These changes improve respiratory acidosis by increasing CO2 elimination, thereby reducing the likelihood of auto-PEEP. For volume-controlled ventilation, flow is not preset and is variable. Flow rate is determined by the inspiratory pressure limit, the inspiratory time, and the compliance of the respiratory system. Mechanical ventilators can deliver varying inspiratory flow patterns, including a square waveform (constant flow), a ramp waveform (decelerating flow), and a sinusoidal wave. Ramp waveforms are more commonly used because they distribute ventilation more evenly, decreasing peak airway pressure, physiologic dead space, and PaCO2 while not altering oxygenation (Hyzy & McSparron, 2024).
- Trigger sensitivity: Breaths can be initiated by patient effort or a timer (ventilator-initiated). If the timer is set to deliver ventilator-initiated breaths, trigger sensitivity does not need to be set. However, patient-triggered breaths occur when the patient causes a sufficient change in pressure (pressure-triggering) or flow (flow-triggering) in the circuit. Therefore, trigger sensitivity must be set. Flow triggering is preferred because it is associated with less inspiratory effort. With flow triggering, the ventilator monitors the continuous flow of gas through the ventilator circuit. When the return flow is less than the delivered flow, a ventilator-delivered breath is initiated. The trigger sensitivity is usually set at 2 LPM, with ventilator-assisted breaths triggered when the patient's inspiratory effort generates a flow of 2 LPM. A ventilator-delivered breath is initiated with pressure triggering if the demand valve senses a negative airway pressure generated by the patient trying to initiate a greater breath than the trigger sensitivity. Ventilator-assisted breaths will be triggered with a sensitivity set at -1 to -3 cm H2O when the alveolar pressure decreases to 1 to 3 cm H2O below atmospheric pressure. Pressure triggering should not be used if auto-PEEP is suspected (Hyzy & McSparron, 2024).
Troubleshooting the Ventilator
Managing patients requiring MV is complex, with numerous ventilator modes and settings for various needs. Therefore, basic ventilator modes, initial ventilator settings, and circumstances for setting variations have been discussed. HCPs should be familiar with these settings and understand ventilator safety considerations. Knowing how to identify and manage ventilator problems can prevent serious complications. The most common issues that need to be addressed with ventilator management are hypoxemia, hypercapnia, elevated peak and plateau pressures, and auto-PEEP (Mora Carpio & Mora, 2023).
Hypoxia
Many patients receiving MV have acute respiratory failure requiring assistance with oxygenation and ventilation. During MV, oxygenation depends on FiO2 and PEEP. Therefore, increasing these parameters should improve hypoxia. However, increasing PEEP too much can cause barotrauma and hypotension, while increasing FiO2 too much can lead to oxidative damage in the alveoli. Setting an appropriate goal for oxygenation—90% to 96%—can prevent excessive oxygenation. HCPs should monitor for a sudden drop in oxygenation, which could indicate ET tube misplacement, PE, pneumothorax, pulmonary edema, atelectasis, or a mucus plug. Prompt identification and management of hypoxia can prevent complications, including circulatory collapse (Mora Carpio & Mora, 2023).
Hypercapnia
Some patients receiving MV will develop elevated CO2 levels. If a patient is experiencing hypercarbia, HCPs must modify alveolar ventilation by increasing the respiratory rate or the TV to increase ventilation and decrease CO2. Increasing the TV should be considered first since it is more efficient at decreasing the CO2 and boosting the respiratory rate, which can increase the amount of dead space. HCPs should monitor patients closely when increasing the respiratory rate or TV due to the risk of developing auto-PEEP (Mora Carpio & Mora, 2023).
Elevated Peak and Plateau Pressure
HCPs must monitor patients receiving MV for elevated peak pressure (airway resistance) and plateau pressure (alveolar pressure and lung compliance). If an increase in peak pressure occurs, plateau pressure should be checked. When plateau pressure is normal and peak pressure is elevated, possible causes can include a kinked ET tube, mucus plug, or bronchospasm. HCPs should troubleshoot these issues by assessing the tube, suctioning the patient, or administering bronchodilators. An elevated peak and plateau pressure indicate a compliance problem. Potential causes of compliance issues and troubleshooting strategies include (Mora Carpio & Mora, 2023):
- Mainstem intubation (i.e., endobronchial intubation, where the ET tube is placed into the left or right mainstem bronchus) should be considered if unilateral breath sounds and a dull contralateral lung (atelectatic lung) are found. HCPs should retract the ET tube if mainstem intubation is confirmed.
- Pneumothorax should be considered if unilateral breath sounds and a hyper-resonant contralateral lung are found. A chest tube is indicated, as positive pressure will worsen a pneumothorax.
- If atelectasis is found, chest percussion should be initiated.
- With pulmonary edema, HCPs should consider diuresis, high PEEP, and inotropes.
- For patients with ARDS, troubleshooting can include using a low TV and high PEEP ventilation.
Ventilator Alarms
All ventilators are equipped with alarms that can alert HCPs to changes in ventilation. Whenever a ventilator alarm occurs, HCPs should immediately investigate the cause of the alarm and check the patient's status. Ventilator alarms should never be ignored or silenced. Most health care institutions have policies and procedures to address this event (usually developed with an interprofessional team, including respiratory therapists). Programming alarms appropriately can improve ventilator safety and patient outcomes. Ventilator alarms can also be used to prevent ventilator settings (rate, pressure, and volume) from exceeding appropriate limits (Williams & Sharma, 2023).
Dynamic Hyperinflation or Auto-PEEP
Auto-PEEP occurs when some of the inhaled air is not fully exhaled at the end of the respiratory cycle. As the trapped air accumulates, pulmonary pressures increase, leading to barotrauma and hypotension. When auto-PEEP occurs, patients become more difficult to ventilate. To prevent or treat auto-PEEP, HCPs must ensure enough time to allow the air to leave the lungs during exhalation. Ventilator setting adjustments should be made to decrease the I:E ratio by reducing the respiratory rate, decreasing the TV (a higher volume will require a longer time to leave the lungs), or increasing the inspiratory flow (if the air is delivered faster, then the inspiratory time will decrease). The same effect can also be achieved using a square waveform (full flow throughout inhalation) for inspiratory flow. Other potential troubleshooting measures can include ensuring adequate sedation (preventing hyperventilation) and administration of bronchodilators or steroids (to decrease airway obstruction; Mora Carpio & Mora, 2023).
Consequences of MV
MV is a standard treatment for critically ill patients who require life-sustaining airway protection, oxygenation, or ventilation. As discussed earlier, the use of MV, including appropriate ventilator modes and settings, can prevent complications from occurring. HCPs must diligently monitor patients for changes in oxygenation and ventilatory status so that troubleshooting can occur promptly. In addition to the complications of MV discussed, HCPs should be aware of additional physiologic and pathophysiologic consequences that can affect patients requiring MV. See Tables 3 and 4 for MV's pulmonary and systemic effects (Hyzy, 2023a).
Table 3
Pulmonary Effects of MV
Mechanism of Action | |
Barotrauma or VLI | Discussed in complications of MV |
Auto-PEEP | Discussed in troubleshooting the ventilator |
Heterogeneous ventilation | The distribution of ventilation is based on alveolar compliance, airway resistance, and dependency within the lungs. Areas of the lungs that are more compliant are less dependent, have minimal resistance, and will be better ventilated. Ventilation heterogeneity is accentuated in patients with airway and parenchymal lung disease; stiffer, dependent lungs; and increased airway resistance. |
V/Q mismatch | V/Q mismatch can occur from two opposing mechanisms: dead space (areas that are overventilated relative to perfusion, V>Q) and shunt (areas that are underventilated relative to perfusion, V<Q). Dead space refers to the alveoli that are not involved in gas exchange due to insufficient perfusion. Positive-pressure ventilation increases dead space, causing V/Q mismatch and hypercapnia. |
Diaphragm | MV causes diaphragmatic muscle atrophy (also known as ventilator-induced diaphragmatic dysfunction), which is associated with PMV, difficulty weaning, prolonged ICU stays, and a higher risk of complications. CMV can cause rapid disuse atrophy within the first day of use. Numerous studies have shown that diaphragmatic strength decreases progressively with the length of time on the ventilator. |
Respiratory muscles | Patients receiving MV can develop respiratory muscle atrophy; generalized neuromuscular weakness is common for critically ill patients. |
Mucociliary motility | Mucociliary motility in the airways is impaired by positive-pressure ventilation, resulting in secretion retention and pneumonia. The use of mucolytics has been poorly studied and is not routinely performed. |
(Hyzy, 2023a)
Table 4
Systemic Effects of MV
Systemic Effect | Mechanism of Action |
Hemodynamics | Positive-pressure ventilation is frequently associated with decreased cardiac output, leading to hypotension. Decreased cardiac output occurs via various mechanisms:
The extent to which these effects occur can vary based on the chest wall and lung compliance. The transmission of airway pressure is greater with low chest wall compliance or high lung compliance (e.g., emphysema). Conversely, airway pressure transmission is smaller in those with high chest wall compliance or low lung compliance (e.g., ARDS, heart failure). |
Gastrointestinal (GI) | Positive-pressure ventilation for over 48 hours increases the risk of GI bleeding due to stress ulceration. Positive airway pressure is also associated with decreased splanchnic perfusion, which results in elevated plasma aminotransferase and lactate dehydrogenase levels. Additional GI complications can include erosive esophagitis, diarrhea, acalculous cholecystitis, and hypomotility. |
Renal | MV increases the risk of acute renal failure, possibly due to the release of inflammatory mediators (interleukin-6) and impaired renal blood flow from decreased cardiac output, increased sympathetic tone, or activation of humoral pathways. |
Central nervous system | Positive-pressure ventilation increases intracranial pressure, likely from elevated intrathoracic pressure impairing cerebral venous outflow. |
Weakness | Systemic muscular weakness—known as ICU-acquired weakness—occurs from immobilization, prolonged use of sedatives, neuromuscular blocking agents, and critical Illness. Early mobilization (EM) can increase the likelihood of a patient returning to independent functional status. |
Immune system | Positive-pressure ventilation can induce the release of inflammatory mediators, leading to systemic inflammation. |
Sleep | Disordered sleep is common among critically ill patients due to environmental, disease-related, and treatment-related factors. In addition, the mode of ventilation, particularly PSV, may influence sleep quality. |
Other | Critically ill patients with prolonged bed rest can experience insulin resistance, venous thromboembolic disease, joint contractures, and pressure injuries. |
(Hyzy, 2023a)
Complications of MV
Patients requiring MV are at high risk for complications and poor outcomes, including death. Ventilator-associated pneumonia (VAP), barotrauma, PE, VLI, sepsis, and ARDS are VAEs that can affect patients receiving MV. These complications can result in PMV, prolonged ICU stays, increased health care costs, and high mortality. Mortality rates for patients with an acute lung injury range from 24% in persons 15 to 19 years to 60% for patients 85 years and older (NHSN, 2024). Before 2013, the NHSN surveillance for VAEs was limited to VAP. However, accurate VAP surveillance was a challenge due to inconsistent VAP definitions, with many requiring radiographic findings of pneumonia. The subjectivity and variability of chest radiographic techniques, interpretation, and reporting make this imaging inappropriate for VAP diagnosis. Another limitation of the VAP surveillance definition is the reliance on specific clinical signs and symptoms, which are subjective and inconsistently documented. In 2013, the NHSN implemented the VAE surveillance definition algorithm based on objective and streamlined criteria to identify a broad range of conditions or complications occurring in mechanically ventilated patients (NHSN, 2024). The CDC defined a VAE as a sustained increase in ventilator support following a period of stable or decreasing ventilator support. This definition broadened the focus from pneumonia alone to encompass all major causes of respiratory deterioration for ventilated patients (e.g., pneumonia, pulmonary edema, ARDS, PE, atelectasis; Klompas, 2019; Patel, 2024b).
In 2016, VAE rates for critical care units nationwide were 6.8 events per 1,000 ventilator days. These rates varied substantially by ICU type, with higher rates in trauma, surgery, and neurological units and lower rates in medical and cardiac units. VAE rates were also higher in teaching hospitals compared to non-teaching hospitals. Although patients are at risk for VAEs until extubation occurs, the highest risk is within the first 2 weeks of MV (specifically days 3 to 7). VAEs are divided into two subcategories to distinguish VAP from other ventilator-associated complications. Infection-related ventilator-associated complications (IVAC) are defined as the presence of an abnormal temperature or white blood cell count and at least 4 days of newly prescribed antibiotics starting within 2 days of VAE onset. Most VAEs are triggered by one of four clinical events: pneumonia (25% to 40% of cases), ARDS (5% to 20% of cases), atelectasis (10% to 15% of cases), and fluid overload (e.g., pulmonary edema; 15% to 50% of cases). Approximately one-third of VAEs meet the IVAC criteria. The overall mortality rate for VAEs is 31%, and patients developing VAEs are twice as likely to die compared to patients who did not develop VAEs. Thus, the prevention of VAEs is critical to optimizing outcomes for patients receiving MV. Strategies for preventing VAEs can include elevating the head of the bed, daily oral care, spontaneous awakening and breathing trials, and EM (Klompas, 2019).
VAP
VAP is a lung infection that develops in mechanically ventilated patients when pathogens enter through the ET tube and reach the lungs (CDC, 2024d). VAP is hospital-acquired pneumonia that develops after 48 hours of MV (Kollef, 2023). Only 40% of VAPs meet the criteria for VAEs, as 60% are clinically diagnosed without sustained increases in ventilator support (Klompas, 2019). According to the NHSN (2024), VAP rates have steadily declined in the US between 2006 and 2012, from 3.1 to 0.9 per 1,000 ventilator days in medical ICUs and from 5.2 to 2.0 per 1,000 ventilator days in surgical ICUs. However, VAP is associated with extended hospital stays, PMV, and significant health care costs ($40,000 per patient). Various pathogens may cause VAP and can be polymicrobial. Some of the most common pathogens include aerobic gram-negative bacilli (e.g., Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa) and gram-positive cocci (e.g., Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae; Klompas, 2023). Therefore, HCPs must be acutely aware of the clinical features associated with VAP.
Most patients with VAP present with sudden or gradual onset of the following clinical features (Kollef, 2023):
- dyspnea (although many patients requiring MV may be unable to report symptoms due to sedation or severity of illness)
- tachypnea, increased or purulent sections, rhonchi, crackles, reduced breath sounds, and fever
- ventilator mechanics that show reduced total volume and increased inspiratory pressures
- laboratory findings that show leukocytosis and worsening hypoxemia
- a chest x-ray that shows a new or worsening infiltrate
As isolated findings, none of these clinical features can be sensitive for or specific to the diagnosis of VAP. Therefore, a chest radiograph should be performed for all patients with suspected VAP to evaluate a new or progressive pulmonary infiltrate. A VAP diagnosis can be made by identifying a pulmonary infiltrate on imaging with clinical findings of infection, as outlined earlier (Kollef, 2023). HCPs should ensure patients requiring MV have the head of the bed elevated at least 30° unless contraindicated to prevent aspiration (Hinkle et al., 2021).
For additional information on the prevention, diagnosis, and management of VAP, please see our Pneumonia NursingCE course.
PMV
PMV increases the risk of pneumonia, barotrauma, tracheal injuries, musculoskeletal deconditioning, and mortality. Strategies such as EM of patients on MV can help prevent PMV and facilitate the weaning process. Prolonged ventilatory dependence can stem from many factors, including respiratory insufficiency, reduced ventilatory capacity, and cardiovascular insufficiency. Respiratory insufficiency is an imbalance between respiratory pump capacity and demands. In addition, PMV can lead to diaphragmatic weakness and atrophy, especially when accompanied by passive modes (e.g., CMV or AC) of ventilation. Other factors contributing to respiratory muscle weakness include malnutrition, immobility, excessive steroids, sedative medications, and a systemic inflammatory response associated with sepsis. Cardiovascular insufficiency (i.e., heart failure) can also increase the risk of PMV. When a patient transitions from MV to spontaneous breathing, there is a decrease in intrathoracic pressure. The resulting shift in pressure raises venous return in the right ventricle, increasing preload and afterload. This greater cardiac workload can raise myocardial O2 demand for patients with coronary artery disease, resulting in ischemia (Fadila et al., 2022; Huang et al., 2022). The following risk factors can increase the risk of PMV:
- history of obstructive pulmonary disease (e.g., COPD)
- history of restrictive pulmonary disease (e.g., neuromuscular disorder)
- neuromuscular disorders
- ICU admission due to pneumonia, ARDS, head trauma, and postoperative intracerebral hemorrhage
- abnormal arterial CO2, serum blood urea nitrogen, serum creatinine, arterial pH, white blood cell count, or body temperature on the first ICU day
- age 85 years and older
- extended inpatient length of stay before ICU admission (Fadila et al., 2022; Huang et al., 2022; King Han, 2023)
Barotrauma
Barotrauma is an example of a VLI caused by high lung inflation pressure, which leads to high transpulmonary pressure, regional lung over-distention, and air leakage. With ventilation, positive pressure is needed to overcome airway resistance, increase airflow, and overcome lung elasticity. Plateau pressure is commonly used as a representative indicator of transpulmonary pressure, measuring lung overdistention. Careful control of inspiratory pressures is a strategy to limit lung distention and prevent barotrauma. High inspiratory pressures can cause alveolar rupture, pneumothorax, pneumomediastinum, and subcutaneous emphysema due to barotrauma or air leakage (Haribhai & Mahboobi, 2022).
Preventing Complications Associated With Mechanical Ventilation
Preventative measures, implemented immediately upon admission to the ICU, can improve mortality rates and clinical outcomes and enhance quality of life after discharge. The ABCDEF bundle of care is an evidence-based approach to preventing complications associated with critical illness and MV (Smith & Rahman, 2023). Traditionally, ICU staff have frequently implemented sedation and restraints to facilitate necessary treatments. However, these can threaten patient dignity and result in physical, cognitive, and psychological harm. The ICU Liberation Collaborative is a real-world quality improvement initiative designed to engage health care facilities in the strategic implementation of an ABCDEF bundle utilizing team-based care. Although the bundle should guide implementation, it is not intended to be a static, rigidly applied protocol. Instead, the bundle can be adapted to institutional preferences and needs. The concepts were designed to assist all ICU programs with varying patient populations (e.g., small and large, community and academic, public and governmental). The ICU Liberation Collaboration quality improvement initiative has been implemented in 76 ICUs across the US (Ely, 2018). Numerous studies involving more than 20,000 patients have found that this bundle is associated with a 68% decrease in the likelihood of hospital death within 7 days and a 50% reduction in ICU readmissions. In addition, the ABCDEF bundle is associated with a 25% to 50% reduction in delirium and coma days, a 60% reduction in physical restraints, and a 40% reduction in discharges to long-term care facilities (SCCM, n.d.). The key components of the ABCDEF bundle consist of the following (Ely, 2018; Mart et al., 2019; Mora Carpio & Mora, 2023; SCCM, n.d.):
- Assess, prevent, and manage pain: Pain is a common occurrence in ICUs that can often go unrecognized and undertreated, becoming a risk factor for the development of delirium. HCPs must actively assess and manage pain to prevent complications. Assessment can include self-reporting, observing behavioral changes, asking the family to help identify pain behaviors, and assuming pain is present. Pain should be assessed frequently in the ICU (every 4 hours) and reassessed (within 1 hour) using validated tools such as the numerical rating scale, behavioral pain scale, or critical-care pain observation tool. The preferred first-line medication is intravenous opioids. Alternative medications (e.g., nonsteroidal anti-inflammatory drugs and gabapentin [Neurontin]) and nonpharmacologic interventions should also be considered to reduce opioid doses. Adequate pain management reduces the risk of delirium and facilitates the performance of other components of the ABCDEF bundle (e.g., EM and breathing trials).
- Both spontaneous awakening trials (SATs) and SBTs: Coordinated and protocolized SATs and SBTs are foundational to liberation from MV. SATs involve the cessation of sedatives and narcotics for patients receiving MV. Daily interruption of sedation can decrease ventilator days, VAP rates, mortality, and other complications such as ICU-acquired weakness (ICUAW) and post-intensive care syndrome (PICS). The routine use of SBTs facilitates ventilator weaning and liberation. Protocolized SBTs reduce ventilator days, length of stay, mortality, and other MV complications. HCPs must complete a safety screen before initiating SATs or SBTs. To begin SBTs, HCPs should reduce ventilator support to a minimum (using PSV). SBTs should be 30 to 120 minutes, and the nurse must monitor closely for respiratory distress. If a patient fails an SBT, previous ventilator settings should be restarted. If a patient has a successful SBT, extubation can be considered. Successful SBT parameters include respiratory rate under 35 BPM, no signs of distress, heart rate under 140/minute and heart rate variability under 20%, O2 saturation above 90%, and SBP between 80 and 180 mm Hg or no more than a 20% change from baseline. For additional information on ventilator weaning, please see our Basics of Ventilator Weaning in the Critical Care Patient NursingCE course.
- Choice of analgesia and sedation: HCPs should use goal-directed sedation and analgesia to reduce the overall medication burden and achieve light sedation. This element focuses on safe and effective medication regimens consistent with the pain, agitation, and delirium guidelines and the pain, agitation/sedation, delirium, immobility, and sleep guidelines. Routine assessments of pain and sedation levels should be done using validated measures. The Richmond Agitation-Sedation Scale and the Riker Sedation-Agitation Scale are recommended. The goal of sedation and analgesia for critically ill patients should be a calm, alert state allowing for patient interaction.
- Delirium assessment, prevention, and management: Delirium is an acute pervasive form of brain failure in critically ill patients that is characterized by waxing and waning confusion. Patients can experience hypoactive (i.e., a reduced level of consciousness with fluctuating attention and awareness) or hyperactive (i.e., increasing levels of agitation with fluctuating attention and awareness) delirium. It is associated with increased mortality and long-term complications (e.g., PICS). HCPs play a critical role in identifying delirium, so screening should occur daily in the ICU. Two of the most common and validated instruments for detecting delirium are the Intensive Care Delirium Screening Checklist and the Confusion Assessment Method for the Intensive Care Unit. Promoting sleep, reducing noise, and providing EM can help minimize the development of delirium.
- EM and exercise: EM is an essential component of the ABCDEF bundle that is often underutilized. Depending on a patient's severity of illness, the safety of EM and physical activity is often a concern for HCPs. Prolonged immobilization can lead to muscle wasting and weakness, PMV, poor functional status, ICUAW, and PICS. For additional information on EM, please see our Early Mobilization in the Mechanically Ventilated Patient NursingCE course.
- Family engagement and empowerment: Patients with critical illness and those requiring MV are often unable to communicate with the care team and their families. Patient-centered care respects individual patient preferences, values, and informed clinical decision-making. Empowering family members to engage in shared decision-making and facilitating open communication improves patient outcomes. Critical illness can lead to significant psychological problems for patients and family members. HCPs who actively engage with patients and family members can prevent serious complications, including delirium, ICUAW, and PICS.
References
Allen, G. B. (2024). Invasive mechanical ventilation in acute respiratory failure complicating chronic obstructive pulmonary disease. UpToDate. Retrieved July 26, 2024, from https://www.uptodate.com/contents/invasive-mechanical-ventilation-in-acute-respiratory-failure-complicating-chronic-obstructive-pulmonary-disease
American Association of Critical Care Nurses. (n.d.). Mechanical ventilation settings. Retrieved August 1, 2024, from https://www.aacn.org/clinical-resources/pulmonary/mechanical-ventilation-settings
American College of Emergency Physicians. (2022). Verification of endotracheal tube placement. https://www.acep.org/patient-care/policy-statements/verification-of-endotracheal-tube-placement
American Thoracic Society. (2020). Mechanical ventilation. https://www.thoracic.org/patients/patient-resources/resources/mechanical-ventilation.pdf
Brown, C. A., III. (2023). The decision to intubate. UpToDate. Retrieved July 26, 2024, from https://www.uptodate.com/contents/the-decision-to-intubate
Centers for Disease Control and Prevention. (2024a). About chronic diseases. National Center for Chronic Disease Prevention and Health Promotion. https://www.cdc.gov/chronic-disease/about
Centers for Disease Control and Prevention. (2024b). Deaths and mortality. https://www.cdc.gov/nchs/fastats/deaths.htm
Centers for Disease Control and Prevention. (2024c). Living with a chronic condition. https://www.cdc.gov/chronic-disease/living-with/index.html
Centers for Disease Control and Prevention. (2024d). Ventilator-associated pneumonia basics. https://www.cdc.gov/ventilator-associated-pneumonia/about
Dirkes, S. M., & Kozlowski, C. (2019). Early mobility in the intensive care unit: Evidence, barriers, and future directions. Critical Care Nurse, 39(3), 33–43. https://doi.org/10.4037/ccn2019654
Ely, E. W. (2018). The ABCDEF bundle: Science and philosophy of how ICU liberation serves patients and families. Critical Care Medicine, 45(2), 321–330. https://doi.org/10.1097/CCM.0000000000002175
Fadila, M., Rajasurya, V., & Regunath, H. (2022). Ventilator weaning. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK430712
Haribhai, S., & Mahboobi, S. K. (2022). Ventilator complications. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK560535
Hickey, S. M., Sankari, A., & Giwa, A. O. (2024). Mechanical ventilation. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK539742
Hinkle, J. L., Cheever, K. H., & Overbaugh, K. (2021). Brunner & Suddarth’s textbook of medical-surgical nursing (15th ed.). Wolters Kluwer.
Hou, P., & Baez, A. A. (2023). Mechanical ventilation of adults in the emergency department. UpToDate. Retrieved July 26, 2024, from https://www.uptodate.com/contents/mechanical-ventilation-of-adults-in-the-emergency-department
Huang, H-Y., Huang, C-Y., & Li, L-F. (2022). Prolonged mechanical ventilation: Outcomes and management. Journal of Clinical Medicine, 11(9), 2451. https://doi.org/10.3390%2Fjcm11092451
Hyzy, R. C. (2023a). Clinical and physiologic consequences of mechanical ventilation: Overview. UpToDate. Retrieved August 8, 2024, from https://www.uptodate.com/contents/clinical-and-physiologic-complications-of-mechanical-ventilation-overview
Hyzy, R. C. (2023b). High-frequency ventilation in adults. UpToDate. Retrieved August 2, 2024, from https://www.uptodate.com/contents/high-frequency-ventilation-in-adults
Hyzy, R. C., & Jia, S. (2024). Modes of mechanical ventilation. UpToDate. Retrieved July 30, 2024, from https://www.uptodate.com/contents/modes-of-mechanical-ventilation
Hyzy, R. C., & McSparron, J. I. (2023). Noninvasive ventilation in adults with acute respiratory failure: Benefits and contraindications. UpToDate. Retrieved July 28, 2024, from https://www.uptodate.com/contents/noninvasive-ventilation-in-adults-with-acute-respiratory-failure-benefits-and-contraindications
Hyzy, R. C., & McSparron, J. I. (2024). Overview of initiating invasive mechanical ventilation in adults in the intensive care unit. UpToDate. Retrieved July 24, 2024, from https://www.uptodate.com/contents/overview-of-initiating-invasive-mechanical-ventilation-in-adults-in-the-intensive-care-unit
Jarrett, N. M., Palmer, L., Lewis, R., Le, T., Free, L., Sabouri, S., Bernacet, A., & Eng, T. (2016). Ventilator weaning (liberation) quality measures pilot test for long-term care hospitals: A summary of findings. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/LTCH-Quality-Reporting/Downloads/Ventilator-Weaning-QM-Pilot-Summary-Report-2016.pdf
King Han, M. (2023). Management and prognosis of patients requiring prolonged mechanical ventilation. UpToDate. Retrieved July 24, 2024, from https://www.uptodate.com/contents/management-and-prognosis-of-patients-requiring-prolonged-mechanical-ventilation
Klompas, M. (2019). Ventilator-associated events: What they are and what they are not. Respiratory Care, 64(8), 953–961. https://doi.org/10.4187/respcare.07059
Klompas, M. (2023). Epidemiology, pathogenesis, microbiology, and diagnosis of hospital-acquired and ventilator-associated pneumonia in adults. UpToDate. Retrieved August 8, 2024, from https://www.uptodate.com/contents/epidemiology-pathogenesis-microbiology-and-diagnosis-of-hospital-acquired-and-ventilator-associated-pneumonia-in-adults
Kollef, M. H. (2023). Clinical presentation and diagnostic evaluation of ventilator-associated pneumonia. UpToDate. Retrieved August 8, 2024, from https://www.uptodate.com/contents/clinical-presentation-and-diagnostic-evaluation-of-ventilator-associated-pneumonia
Lutz, E. (2020). Biology of ventilation [Image]. https://commons.wikimedia.org/wiki/File:Biology_of_ventilation.gif
Mart, M. F., Brummel, N. E., & Ely, E. W. (2019). The ABCDEF bundle for the respiratory therapist. Respiratory Care, 64(12), 1561–1573. https://doi.org/10.4187/respcare.07235
Mercury Medical. (2014). Continuous positive airway pressure (CPAP). https://www.mercurymed.com/wp-content/uploads/5Ws-CPAP-Booklet.pdf
Meyers, M., Rodrigues, N., & Ari, A. (2019). High-frequency oscillatory ventilation: A narrative review. Canadian Journal of Respiratory Therapy, 55, 40–46. https://doi.org/10.29390/cjrt-2019-004
Mora Carpio, A. L., & Mora, J. I. (2023). Ventilator management. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK448186
National Healthcare Safety Network. (2024). Ventilator-associated event (VAE). Centers for Disease Control and Prevention. https://www.cdc.gov/nhsn/pdfs/pscmanual/10-vae_final.pdf
Patel, B. K. (2024a). Noninvasive positive pressure ventilation (NIPPV). https://www.merckmanuals.com/professional/critical-care-medicine/respiratory-failure-and-mechanical-ventilation/noninvasive-positive-pressure-ventilation-nippv
Patel, B. K. (2024b). Overview of mechanical ventilation. https://www.merckmanuals.com/professional/critical-care-medicine/respiratory-failure-and-mechanical-ventilation/overview-of-mechanical-ventilation
Pinto, V. L., & Sharma, S. (2023). Continuous positive airway pressure. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK482178
Potchileev, I., Doroshenko, M., & Mohammed, A. N. (2023). Positive pressure ventilation. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK560916
Powers, K. A., & Dhamoon, A. S. (2023). Physiology, pulmonary ventilation and perfusion. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK539907
Respiratory Therapy Zone. (2024). Respiratory therapy normal values: Reference guide. https://www.respiratorytherapyzone.com/respiratory-therapy-normal-values
Smith, S., & Rahman, O. (2023). Post intensive care syndrome. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK558964
Society of Critical Care Medicine. (n.d.). ICU liberation bundle (A-F). Retrieved August 8, 2024, from https://www.sccm.org/iculiberation/abcdef-bundles
Society of Critical Care Medicine. (2024). Critical care statistics. https://www.sccm.org/Communications/Critical-Care-Statistics
Villalba, D., Rossetti, G. G., Scrigna, M., Collins, J., Rocco, A., Matesa, A., Areas, L., Golfarini, N., Pini, P., Hannun, M., Boni, S., Grimaldi, S., Pedace, P., Diaz-Ballve, L., Andreu, M., Bunirigo, P., Noval, D., & Planells, F. (2019). Prevalence of and risk factors for mechanical ventilation reinstitution in patients weaned from prolonged mechanical ventilation. Respiratory Care, 65(2), 210–216. https://doi.org/10.4187/respcare.06807
Williams, L. M., & Sharma, S. (2023). Ventilator safety. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK526044
Wunsch, H. (2020). Mechanical ventilation in COVID-19: Interpreting the current epidemiology. American Journal of Respiratory and Critical Care Medicine, 202(1), 1–21. http://doi.org/10.1164/rccm.202004-1385ED
Yarrarapu, S. N. S., Saunders, H., & Sanghavi, D. (2023). Average volume-assured pressure support. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK560600